The pituitary gland is the master gland of the body because it controls most of the body's endocrine functions by means of the hypothalamic-pituitary axis (see the images below). The anterior lobe of the pituitary gland secretes 6 hormones: thyroid-stimulating hormone (TSH), previously adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), leuteinizing hormone (LH), growth hormone (GH), and prolactin (PRL). The posterior pituitary gland secretes vasopressin and oxytocin.
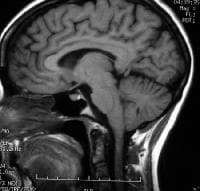 T1-weighted sagittal MRI through the pituitary fossa shows a normal, isointense anterior pituitary and a hyperintense posterior pituitary gland.
T1-weighted sagittal MRI through the pituitary fossa shows a normal, isointense anterior pituitary and a hyperintense posterior pituitary gland.  Lateral skull radiograph in a patient with pituitary adenoma shows an enlarged sella and focal calcification in the adenoma (arrow) Pituitary adenomas are almost always benign with no malignant potential. In general, pituitary lesions can be subdivided into nonsecretory and secretory tumors of the pituitary gland, other intrasellar tumors, and parasellar tumors. The last group occurs in the vicinity of the sellar turcica and can mimic the pituitary tumors in terms of the symptoms they cause. Nonsecretory pituitary tumors are called null-cell tumors. Small null-cell tumors measuring a few millimeters are common and found in up to 25% of autopsied pituitary glands. These may grow slowly, destroying normal pituitary function (hypopituitarism), or they may compress nearby structures and cause neurologic problems.
Lateral skull radiograph in a patient with pituitary adenoma shows an enlarged sella and focal calcification in the adenoma (arrow) Pituitary adenomas are almost always benign with no malignant potential. In general, pituitary lesions can be subdivided into nonsecretory and secretory tumors of the pituitary gland, other intrasellar tumors, and parasellar tumors. The last group occurs in the vicinity of the sellar turcica and can mimic the pituitary tumors in terms of the symptoms they cause. Nonsecretory pituitary tumors are called null-cell tumors. Small null-cell tumors measuring a few millimeters are common and found in up to 25% of autopsied pituitary glands. These may grow slowly, destroying normal pituitary function (hypopituitarism), or they may compress nearby structures and cause neurologic problems. Functioning pituitary adenomas can be clinically classified by means of the hormone they elaborate. These tumors become symptomatic because they secrete hormones, and they are less likely than like null-cell tumors to become large enough to compress adjacent structures. As pituitary tumors grow, destruction of normal pituitary tissue results in various hormonal deficiencies. In rare cases, these tumors may spontaneously hemorrhage or become infarcted. The pressure they exert on nearby structures can produce double vision and facial numbness. The optic chiasm is directly above the pituitary gland, and upward growth of pituitary tumors frequently causes progressive visual loss. This visual loss typically begins from each side of the field of vision and leads to tunnel vision and then blindness.[8, 9]
Clinical features
Features based on secretory abilityThe clinical features of pituitary adenoma vary depending on the location and size of the tumor and its secretory capability. Pituitary adenomas typically appear during early adulthood, and no sex predilection is known. Secretory pituitary adenomas are usually small and generally do not cause neurologic symptoms or hypopituitarism, though they can. The symptoms of functioning tumors are related to the specific hormone the tumor produces.[10, 11]
Neurologic symptoms of pituitary adenomas include headaches; double vision; and loss of peripheral vision leading to blindness, facial pain, or numbness. Hypopituitarism manifests itself by lack of energy, weight loss, nausea, vomiting, constipation, amenorrhea and infertility, dry skin, increase pigmentation of the skin, cold intolerance, and mental status changes (eg, sleepiness, psychosis, collapse).
A prolactinoma is the most common pituitary tumor and may cause amenorrhea, irregular periods, galactorrhea, infertility in women and osteoporosis. It may cause hypogonadism, loss of libido, and impotence in men.
Tumors that secrete excess GH cause gigantism in children and acromegaly in adults. Acromegaly is associated with enlargement of facial features, hands and feet, heart disease, hypertension, arthritis, carpel tunnel syndrome, amenorrhea, and impotence.
ACTH-secreting adenomas produce Cushing disease, which itself results in a widened face with acne and flushing, fatty deposits over the back of the neck, stretch marks, easy bruising, hair growth, diabetes mellitus, muscle loss, fatigue, depression, and psychosis.
Tumors that elaborate TSH produce signs and symptoms of thyrotoxicosis, such as heat intolerance, sweating, tachycardia, fine tremor, and weight loss. Some tumors may secrete more than one hormone, such as GH and PRL.
Rare tumors secrete LH or FSH (gonadotrophins). When pituitary tumors compromise the secretory cells, the first evidence of cellular failure usually affects the gonadotrophins. Therefore, the disappearance of menstrual periods may be the first sign of a pituitary tumor in female patients. In male patients, the most common symptom of deficiency is impotence. Isolated deficiencies of both LH and FSH do occur, but only rarely. In a male individual, LH deficiency alone leads to the appearance of a fertile eunuch. In this condition, sufficient FSH is present to permit the maturation of spermatozoa; however, because of the LH deficiency, the patient has many of the characteristics of a castrated individual. Tumors also can produce an excess of LH or FSH, and pituitary tumors that secrete only the nonspecific, hormonally inactive alpha unit of glycoprotein hormones are not rare.
Other features
Visual symptoms are generally related to compression of visual pathways and include bitemporal visual-field loss, which is denser from the superior to inferior than in other orientations, color desaturation, diplopia, and ophthalmoplegia. The funduscopic sign of long-standing chiasmal compression is primary optic atrophy. Severe optic atrophy indicates a poor prognosis for visual recovery after surgical decompression. In pregnant women, bitemporal visual-field loss and headache may indicate pituitary apoplexy.
Pituitary apoplexy is a potentially life-threatening condition. Women with pituitary adenomas and MRI evidence of subarachnoid bleeding should deliver by cesarean section to prevent apoplexy during delivery. Postpartum hemorrhage can cause infarction of the pituitary gland, leading to hypopituitarism (Sheehan syndrome).
Other problems to consider
Clinical masqueradesClinical masquerades of pituitary adenoma include chronic retrobulbar optic neuritis, nutritional amblyopia, uncorrected refractive error, normal-tension glaucoma, and age-related maculopathy. Bilateral tilted-disc syndrome can result in a superior bitemporal field defect similar to that observed in pituitary adenoma. However, the field defect in tilted-disc syndrome is unchanging and does not respect the vertical midline, whereas the field defects in chiasm-compressive lesions are progressive and do respect the vertical midline.
Craniopharyngioma
Craniopharyngiomas are benign, slow-growing tumors that originate from epithelial remnants of the Rathke pouch at the junction of the infundibulum and the pituitary gland. These lesions are composed of both solid epithelial tissue and cystic components. The cystic components contain variable amounts of cholesterol, keratin, necrotic debris, proteinaceous fluid, and hemorrhage. Calcification is present in 75-85%.
MRI appearances vary depending on the amount of solid and cystic components and on the nature of cystic contents. Heterogeneous signal intensity on images obtained with all sequences is the most typical finding. Solid components are hypointense on T1-weighted images and hyperintense on T2-weighted images. The cysts also have a long T2; however, if they have a high cholesterol content or methemoglobin, shortening of T1 results in high signal intensity on T1-weighted images. Calcification in the tumor is better detected with CT than with MRI because MRI may not reliably depict calcification. Craniopharyngioma may also cause truncation of the dorsum sellae and upward growth into the third ventricle, which is readily identified on MRI.
Aneurysm
Aneurysms in the parasellar region may originate from the circle of Willis or intracavernous carotid arteries. MRI features include a mass of heterogeneous signal intensity due to flow effects and thrombus formation. Low signal intensity is caused by high flow and chronic thrombus; high signal intensity may represent slow flow or subacute thrombus. Flow in the patent lumen may also cause a band of artifact in the phase-encoding direction on spin-echo images. Magnetic resonance angiography (MRA) is useful in confirming an aneurysm. A potential pitfall in diagnosis is a pneumatized anterior clinoid or calcification, which can simulate the flow void of an aneurysm.
Empty Sella
An empty sella occurs as a result of herniation of the arachnoid through an incompetent diaphragma sellae. Over time, cerebrospinal fluid (CSF) pulsations may enlarge the sella and compress the gland against the floor of the sella. An empty sella is usually asymptomatic and an incidental finding, but can be a manifestation of increased intracranial pressure. However, they are occasionally severe. Compression of the pituitary gland may affect function, or traction on the optic chiasm and nerves may cause visual symptoms.
Chiasmatic and hypothalamic gliomas
Gliomas of the optic chiasm and the hypothalamic pathways are primarily tumors of children and young adults. The tumors tend to be low grade, but they infiltrate along the visual pathways. Neurofibromatosis is strongly associated with optic and chiasmatic gliomas. Hypothalamic gliomas are generally aggressive and produce symptoms early, resulting in 1 of many hypothalamic syndromes: diabetes insipidus; inappropriate secretion of antidiuretic hormone (ADH); Fröhlich syndrome; or disturbances of temperature, appetite, or metabolism.
Chiasmatic gliomas are usually isointense or slightly hypointense on T1-weighted images and hyperintense on T2-weighted images. MRI is useful in determining degree of infiltration of the optic chiasm or optic nerves and for assessing posterior extension into the lateral geniculate body and the occasional exophytic growth into the suprasellar and interpeduncular cisterns. Both hypothalamic and chiasmatic gliomas are enhancing after the intravenous administration of gadolinium-based contrast agent. The multiplanar capability of MRI enables it to depict extension into surrounding structures well.
Pituicytomas
Pituicytomas are also called choristomas, granular cell tumors, or myoblastomas and are rare largely noninfiltrating sellar or parasellar tumors in adults. They arise along the distribution of the neurohypophysis, including both the stalk and the posterior lobe. They occur in the suprasellar space, in the sella, or both. Most symptomatic pituicytomas appear as suprasellar masses. They may originate from the posterior pituitary and remain confined within the sella turcica. Pituicytoma is generally a surprise finding in that it is seldom considered in the preoperative differential diagnosis of a suprasellar lesion. The MRI signal-intensity characteristics vary depending on the cystic components. The solid parts of the tumor are enhancing.
Ectopic pituitary
An ectopic pituitary gland results in high signal intensity adjacent to the median eminence of the hypothalamus with an absence of the normal posterior pituitary bright spot on T1-weighted MRIs. An ectopic pituitary may be associated with perinatal asphyxia and disruption of the normal hypothalamic-pituitary axis. Traumatic transection of the stalk is exceptionally rare, but it can result in abnormal accumulation of posterior-lobe hormones proximal to the disruption.
Metastasis
Most metastases to the pituitary gland are small, clinically silent, and rare in the clinical setting, though they are frequently reported in autopsy series. Large metastasis may cause diabetes insipidus. Leukemia, lymphoma, and cancers of the lung or breast are the most common primary origins. The demonstration of rapid growth distinguishes metastases from slow-growing pituitary adenomas.
Rathke cleft cyst
Rathke cleft cysts arise from remnants of the Rathke cleft, a fetal link between the hypothalamus and nasopharynx that obliterates in normal individuals. Rathke cleft cysts are benign cysts lined by a single layer of ciliated columnar or cuboidal epithelium, and they often contain goblet cells. When small, Rathke cleft cysts are intrasellar. However, as they grow, they extend into the suprasellar region. Rathke cleft cysts are smoothly marginated and well-defined lesions. Their MRI signal-intensity characteristics vary depending on the contents of the cyst. After gadolinium enhancement, only the capsule is enhancing; the nodular component is not enhancing. This feature helps distinguish these lesions from craniopharyngiomas.
Hamartoma of the tuber cinereum
Hamartomas of the tuber cinereum of the hypothalamus are benign, slow-growing tumors that consist of hyperplastic hypothalamic glial and neural tissue. The usual presentation is precocious puberty. The tumors may be sessile or pedunculated, occurring between the infundibulum and mamillary bodies. They are usually isointense to the brain on T1-weighted images and mildly hyperintense on T2-weighted images. They do not show contrast enhancement, a feature that distinguishes them from hypothalamic gliomas and germinomas.
Lymphoma
Favored sites for primary malignant non-Hodgkin lymphomas are the hypothalamus, the cavernous sinuses, and the perisellar regions. These rapidly growing tumors mostly affect patients who are immunocompromised because of chemotherapy, HIV infection, or organ transplantation. Lymphomas typically appear as homogeneous, slightly hyperintense masses on T2-weighted images. In general, lymphomas are uniformly and intensely enhancing. Cystic, hemorrhagic, and necrotic areas in these tumors are unusual.
Germinoma
Germinomas occur in the pineal and suprasellar region and affect children and young adults. Because of their propensity to invade the hypothalamus and to grow into the third ventricle, they may cause endocrine dysfunction. They are known to disseminate through CSF pathways. The tumors are usually well defined and isointense with the brain on T1- and T2-weighted images. They generally do not show necrosis, cystic change, or hemorrhage. Contrast enhancement is moderate and essential in the assessment of CSF spread of the tumor. Calcification in germinomas may make them difficult to visualize on MRIs.
Arachnoid cyst
Arachnoid cysts are CSF-containing spaces that are generally not confused with pituitary or parasellar tumors. These cysts have CSF signal intensity, they are well defined, they do not calcify, and they are not enhancing after the administration of contrast material.
Epidermoid cyst
Epidermoid cysts are benign, slow-growing tumors that arise from epithelial cell rests in the basal cisterns. They have a propensity to grow along the subarachnoid spaces and into the various crevices at the base of the brain. Intradural epidermoids are usually large, with lobulated outer margins and an insinuating pattern of growth. Epidermoid cysts are classically confused with arachnoid cysts on CT and MRI because they are similar to CSF in attenuation and intensity, respectively, and because they have similar T1 and T2 signal intensities. Diffusion-weighted images, proton density–weighted images, and fluid-attenuated inversion recovery (FLAIR) images are useful in making the distinction. Epidermoid cysts do not show contrast enhancement.
Dermoid cyst
Dermoid cysts occur in the pineal and suprasellar regions, as well as other midline locations. On histology, they have both mesodermal and ectodermal derivatives, which account for their varied appearance on MRI. The MRI appearances are those of a heterogeneous tumor. Fatty tissues in the tumor produce high signal intensity on T1-weighted images, and a fat-fluid level may be seen. These cysts may rupture, causing the cystic contents to leak into a ventricle or subarachnoid space and then produce an ependymitis or meningitis, respectively.
Meningioma
Parasellar meningiomas commonly involve the cavernous sinus and produce ophthalmoplegia. Meningiomas are hypervascular tumors that derive their blood supply from the dural vessels. They also induce an osteoblastic reaction in the adjacent bone, resulting in a characteristic focal hyperostosis, which is well depicted on plain radiographs and CT. MRI signal is isointense relative to the brain on T1- and T2-weighted images. MRI is particularly good for assessing encasement of the cavernous sinus and carotid artery. The tumor is intensely enhancing on contrast-enhanced images.
Schwannomas
Schwannomas are nerve-sheath tumors that may involve cranial nerves III-XII or peripheral nerves. Schwannomas in the parasellar region arise from the trigeminal nerve and, in rare cases, from the third, fourth, or sixth cranial nerves. These tumors are benign, well encapsulated, and globular; all of these features that distinguish them from broad-based meningiomas. The tumors are isointense on T1-weighted MRIs and mildly hyperintense on T2-weighted MRIs. Cystic degeneration is frequent; hemorrhage and calcification are rare. The solid portions of these tumors are strongly enhancing with the use of intravenous contrast material.
Primary and secondary tumors of the skull base
A wide variety of primary and metastatic tumors of the skull base can involve the parasellar region. Nasopharyngeal carcinomas and malignant tumors of the paranasal sinuses may invade the parasellar region, and metastases from distant primary tumors may also involve the sphenoid bone and the parasellar region. The primary tumors include chordoma, chondroma, chondrosarcoma, and plasmacytoma.
Chordomas have uniform high signal intensity on T2-weighted MRIs. Chondrosarcomas are heterogeneous on images obtained with all sequences because of their variable calcification and chondral elements, but they usually have areas of high T2 signal intensity. Chordomas characteristically arise in the midline, whereas chondrosarcomas arise off midline at the synchondroses. Alteration of the normal hyperintense clival fat is a sensitive indicator of these tumors; therefore, nonenhanced non–fat-saturated T1-weighted imaging is particularly useful. Contrast enhancement is helpful in assessing the intracranial components of these tumors.
Carotid-cavernous fistula
The fistulous communication between the carotid artery and cavernous sinus may result from a dural arteriovenous malformation, trauma, or transsphenoidal surgery. Arterial pressures cause expansion of the cavernous sinus and dilation of parasellar and orbital veins. Proptosis, chemosis, and visual loss may complicate such fistulas. Flow effects and flow artifacts on MRI may confirm the diagnosis.
Granulomatous hypophysitis
Granulomatous hypophysitis is a rare entity that mimics a pituitary adenoma both clinically and radiologically. The reported causes of granulomatous hypophysitis include tuberculosis, syphilis, sarcoidosis, mycotic granuloma, and foreign body granuloma due to a ruptured Rathke cleft cyst. Lymphocytic hypophysitis represent inflammation of the pituitary gland and may complicate pregnancy or the postpartum period. MRI findings include diffuse enlargement of the anterior lobe and pituitary stalk. The gland is strongly enhanced with gadolinium-based contrast agents, and the enhancement commonly extends along the diaphragma sellae.
McCune-Albright syndrome
McCune-Albright syndrome (MAS) may clinically mimic acromegaly or gigantism. The syndrome is characterized by polyostotic fibrous dysplasia, café au lait pigmentation of the skin, and autonomous endocrine hyperfunction. The most common form of autonomous endocrine hyperfunction in this syndrome is gonadotropin-independent precocious puberty; however, affected individuals also may have hyperthyroidism, hypercortisolism, pituitary gigantism, or acromegaly. Nonendocrine abnormalities in this disorder include hypophosphatemia, chronic liver disease, tachycardia, and sudden death (which is rare and possibly due to cardiac arrhythmias).
Preferred examination
The clinical diagnosis of pituitary adenomas depends on the combination of symptoms and signs resulting from the size of the tumor and/or the type of hormone produced. CT and MRI have largely replaced plain radiography because conventional radiography is poor for delineating soft tissues.[1, 2, 3, 4, 5, 6, 7]Angiography is seldom performed; if indicated, CT angiography (CTA) and MRA have largely replaced conventional angiography. Angiography has a role when intervention is indicated in the cavernous sinus or in the cavernous part of the carotid artery.
Conventional single-section CT has a limited role in pituitary imaging, with a sensitivity of 17-22% in detecting microadenomas. Multidetector-row CT with 64 channels may have a role, especially in patients unable to undergo MRI. CT is best for visualizing bony detail and calcification in tumors such as germinomas, craniopharyngiomas, and meningiomas. CTA is excellent for showing the morphology of parasellar aneurysms and for presurgical planning. CT scans are valuable when MRI is contraindicated, as in patients with pacemakers or metallic implants in the brain or eyes.
MRI is generally preferred over CT for the diagnosis of pituitary adenomas because of its superior definition of small lesions in the pituitary sella and its improved anatomic definition before surgery. MRI is also preferred for postsurgical surveillance.
Somatostatin-receptor scintigraphy may be used to distinguish recurrent or residual tumor from scar or necrotic tissue after surgery.
Limitations of techniques
Conventional radiographs are poor for delineating soft tissues. MRI is more expensive than CT, but it is the preferred imaging study for the pituitary because it improves visualization of the soft tissues and vascular structures. Other limitations of CT include suboptimal imaging of the soft tissues compared with MRI, the need for intravenous contrast medium to enhance images, and the exposure to radiation.A potential pitfall of MRI diagnosis is a pneumatized anterior clinoid or calcification, which can simulate the flow void of an aneurysm. In addition, MRI is contraindicated in patients with pacemakers or ferromagnetic implants in the brain or eyes. With CT or MRI, pituitary adenoma remnants can be hard to differentiate from radiotherapy-induced fibrosis, especially in patients with clinically nonfunctioning pituitary adenomas, who lack circulating markers that aid in monitoring the progression or cure of the disease.
Hiç yorum yok:
Yorum Gönder