To contact us Click HERE
Radiography is an imaging technique that produces high-quality anatomic images by using x-rays. General radiography is currently a major part of hospital imaging departments and includes abdomen, chest and extremity examinations.The use of digital radiography has rapidly increased in recent years. Computed radiography provided a cost-effective transition mode from the traditional film (from the year 1895) to the direct digital radiography (DDR), by using conventional x-ray equipment. Direct digital radiography is a cassette-less imaging system and is ideal for applications where high throughput is of primary importance. The direct digital radiography system should allow usage of all general radiography diagnostic applications.The major components of a digital radiography system are as follows:
1. X-ray generator
2. X-ray assembly
3. Table trolley or other device to support the patient
4. Support for the x-ray tube assembly
5. Detector which converts the x-rays to an image
6. Acquisition workstation to process and display the imageThere is a variety of technologies on which the direct digital radiography is based:
1) Indirect conversion detector: x-rays are converted to light scintillations and light is converted to electric signals.
2) Direct Conversion Detector: x-rays are directly converted to electric signals.
3) Linear Scanning Detectors: A fan beam of x-ray scans the examined area synchronously with a slot of detectors.Due to the structure of the detectors, indirect and direct x-ray conversion detectors are frequently referred to as flat panel detectors (FPD's). There are also portable digital cassettes available, either sold as part of a system or can be retrofitted to an existing CR or film/screen room. Portable detectors can be used in conjunction with an x-ray mobile unit. Such detectors can be connected to a review work station by wire or via a radio link.Most of the digital detectors will need some level of environmental control. This may be in terms of operating temperature range, rate of exchange of temperature and/ or relative humidity.As the original image from the detector is likely to be unsuitable for operating; image processing needs to be applied. A flat field correction is usually applied to the raw image to account for variations in the detector sensitivity across its full area. Also, a number of individual pixels may be defective.The majority of direct digital radiography units are provided with automatic exposure control (AEC) to provide the selected dose to the detector. This may use a conventional AEC detector or the actual image detector to determine the correct dose level. It is essential that the AEC operates in a reliable and consistent manner and that it is correctly set up for the detector of the exposure.Optimization is the process of identifying the necessary radiation dose level to provide adequate clinical information for a particular examination. Optimization depends on a range of both clinical and technical factors.In modern digital radiography systems there is an inbuilt detector dose indicator. The detector dose indicator (DDI) gives an indication of the level of radiation exposure received by the detector. This is useful for monitoring that the exposure is in the correct range for optimal image quality and for undertaking QA.MedWOW.com - an innovative Medical Equipment marketplace for new and used medical equipment. To learn more about Digital Radiography and Medical Radiography Equipment please visit MedWOW.comArticle Source: http://EzineArticles.com/?expert=Bill_Pere
Article Source: http://EzineArticles.com/5396824
25 Haziran 2012 Pazartesi
Diagnostic Radiology - The Advanced Imaging Techniques to Diagnose Fatal Disease
To contact us Click HERE
Diagnostic radiology includes the technique and process that are used to generate images of the human body for the clinical purposes. There are certain medical procedures that are used to reveal, diagnose or examine disease. The imaging of the organs and tissues can be performed as a part of radiotherapy by which the images of the organs and tissues are generated to detect the problem in a better way.
Basically, physicians perform two forms of radiographic images, such as projection radiography and fluoroscopy. These two-dimensional techniques cost low in comparison to the 3D tomography. In the wide sense this biological imaging incorporates radiology, nuclear medicine, endoscopy, investigative radiological sciences, thermography, medical photography and microscopy.
Fluoroscopy generates real-time images of internal structures of the body by employing a constant input of X-rays at a lower dose rate. On the other hand projectional radiographs, known as X-ray, are used to determine the type and extent of the damaged bone or fractures as well as they help to detect pathological changes in the lungs.
There is certain imaging techniques used under the diagnostic radiology. The popular techniques are Magnetic resonance imaging (MRI) scan, Computed tomography (CT) scan, X-ray, Ultrasound. Physicians can use radiographic methods for extensive clinical purposes, such as-
Cardiovascular radiology- it is used to diagnose the diseases of the heart and blood vessels. Physicians perform X-ray, CT, MRI and ultrasound for under this treatment procedure.
Breast imaging- this imaging technology can be used for the diagnosis of breast diseases and conditions. Here doctors can perform mammography, breast ultrasound, breast MRI and breast biopsy to heal breast cancers.
Chest radiology- this stream of radiology is devoted to diagnose the diseases related to chest, such as heart and lung cancers. It takes the help of X-ray, ultrasound, MRI, CT and chest procedures.
Gastrointestinal (GI) radiology- this branch of radiology is used for the imaging and diagnosis of the gastrointestinal (or digestive tract) and abdomen. The CT scan, X-ray, MRI, GI procedures are very useful for such biopsy.
Head and neck radiology- this type of radiology is used for the imaging and diagnosis of the head and neck diseases. It includes several radiographic technologies including CT (or CAT), MRI, ultrasound, X-ray.
There are some other forms of diagnostic radiology, such as emergency radiology, genitourinary radiology, musculoskeletal radiology, neuroradiology, pediatric radiology, interventional radiology, radiation oncology, nuclear radiology, etc.
For more information about radiology, treatment of cancer, diagnostic radiology, and computed tomography please visit radiology-info.org
Article Source: http://EzineArticles.com/?expert=Rebecca_Brown
Article Source: http://EzineArticles.com/3963800
Basically, physicians perform two forms of radiographic images, such as projection radiography and fluoroscopy. These two-dimensional techniques cost low in comparison to the 3D tomography. In the wide sense this biological imaging incorporates radiology, nuclear medicine, endoscopy, investigative radiological sciences, thermography, medical photography and microscopy.
Fluoroscopy generates real-time images of internal structures of the body by employing a constant input of X-rays at a lower dose rate. On the other hand projectional radiographs, known as X-ray, are used to determine the type and extent of the damaged bone or fractures as well as they help to detect pathological changes in the lungs.
There is certain imaging techniques used under the diagnostic radiology. The popular techniques are Magnetic resonance imaging (MRI) scan, Computed tomography (CT) scan, X-ray, Ultrasound. Physicians can use radiographic methods for extensive clinical purposes, such as-
Cardiovascular radiology- it is used to diagnose the diseases of the heart and blood vessels. Physicians perform X-ray, CT, MRI and ultrasound for under this treatment procedure.
Breast imaging- this imaging technology can be used for the diagnosis of breast diseases and conditions. Here doctors can perform mammography, breast ultrasound, breast MRI and breast biopsy to heal breast cancers.
Chest radiology- this stream of radiology is devoted to diagnose the diseases related to chest, such as heart and lung cancers. It takes the help of X-ray, ultrasound, MRI, CT and chest procedures.
Gastrointestinal (GI) radiology- this branch of radiology is used for the imaging and diagnosis of the gastrointestinal (or digestive tract) and abdomen. The CT scan, X-ray, MRI, GI procedures are very useful for such biopsy.
Head and neck radiology- this type of radiology is used for the imaging and diagnosis of the head and neck diseases. It includes several radiographic technologies including CT (or CAT), MRI, ultrasound, X-ray.
There are some other forms of diagnostic radiology, such as emergency radiology, genitourinary radiology, musculoskeletal radiology, neuroradiology, pediatric radiology, interventional radiology, radiation oncology, nuclear radiology, etc.
For more information about radiology, treatment of cancer, diagnostic radiology, and computed tomography please visit radiology-info.org
Article Source: http://EzineArticles.com/?expert=Rebecca_Brown
Article Source: http://EzineArticles.com/3963800
Medical Radiography Careers
To contact us Click HERE
Medical Radiographers are professionals who use complicated imaging apparatus to x-ray various parts of the human body and assist a thorough diagnosis. They execute medical imaging actions to diagnose medical problems. They are also responsible for preparing patients for radiology examinations, positioning them properly under the machines and ensuring accurate doses of radiation. They also have to maintain patient records and radiographic apparatus.
They find employment in medical practitioner offices, clinics, hospitals and diagnostic imaging centers. Their earning ranges from $18.00 to $24.50 per hour. The medical radiography field over the next few years is expected to grow by leaps and bounds. An estimated job opening of 200 vacancies every year is expected. This is because radiography is assuming important proportions in the diagnostic field. It is almost impossible to diagnose a disease without use of radiography.
The American Registry of Radiology Technologists, ARRT, administer certifying exams for Radiology Technologists. In the Arizona State, a certificate from Medical Radiology Technology Board of Examiners (MRTBE) is necessary for employment. Gate Way community college is one of the colleges offering courses in medical radiography. It offers an associate in applied science degree in medical radiography. The degree not only teaches basic imaging principles, but also prepares the student with the job skills necessary for service. After doing a foundation course in medical radiography the student can further advance his career prospects in other imaging professions such as diagnostic medical ultrasound, nuclear medicine technology and magnetic resonance imaging. Apollo College, Colorado Technical University are among many schools and colleges that offer degree or certificate programs resulting in a career as an X Ray technician, Radiographer or a Radiology Technologist.
Thus, medical radiography is a wise career choice today. It ensures recognition in the field of medicine, since no diagnosis is complete without radiographic evidence.
Medical Careers [http://www.e-MedicalCareers.com] provides detailed information on Medical Careers, Top Medical Careers, Medical Billing Careers, Medical Coding Careers and more. Medical Careers is affiliated with Medical Malpractice Law [http://www.e-medicalmalpractice.com].
Article Source: http://EzineArticles.com/?expert=Eric_Morris
Article Source: http://EzineArticles.com/408869
They find employment in medical practitioner offices, clinics, hospitals and diagnostic imaging centers. Their earning ranges from $18.00 to $24.50 per hour. The medical radiography field over the next few years is expected to grow by leaps and bounds. An estimated job opening of 200 vacancies every year is expected. This is because radiography is assuming important proportions in the diagnostic field. It is almost impossible to diagnose a disease without use of radiography.
The American Registry of Radiology Technologists, ARRT, administer certifying exams for Radiology Technologists. In the Arizona State, a certificate from Medical Radiology Technology Board of Examiners (MRTBE) is necessary for employment. Gate Way community college is one of the colleges offering courses in medical radiography. It offers an associate in applied science degree in medical radiography. The degree not only teaches basic imaging principles, but also prepares the student with the job skills necessary for service. After doing a foundation course in medical radiography the student can further advance his career prospects in other imaging professions such as diagnostic medical ultrasound, nuclear medicine technology and magnetic resonance imaging. Apollo College, Colorado Technical University are among many schools and colleges that offer degree or certificate programs resulting in a career as an X Ray technician, Radiographer or a Radiology Technologist.
Thus, medical radiography is a wise career choice today. It ensures recognition in the field of medicine, since no diagnosis is complete without radiographic evidence.
Medical Careers [http://www.e-MedicalCareers.com] provides detailed information on Medical Careers, Top Medical Careers, Medical Billing Careers, Medical Coding Careers and more. Medical Careers is affiliated with Medical Malpractice Law [http://www.e-medicalmalpractice.com].
Article Source: http://EzineArticles.com/?expert=Eric_Morris
Article Source: http://EzineArticles.com/408869
Brain Imaging in Multiple Sclerosis
To contact us Click HERE
Magnetic resonance imaging (MRI) of the brain is useful in the diagnosis and treatment of multiple sclerosis (MS), an inflammatory, demyelinating condition of the central nervous system (CNS) that is generally considered to be autoimmune in nature. White matter tracts are affected, including those of the cerebral hemispheres, infratentorium, and spinal cord. MS lesions, known as plaques, may form in CNS white matter in any location; thus, clinical presentations may be diverse. Continuing lesion formation in MS often leads to physical disability and, sometimes, to cognitive decline.
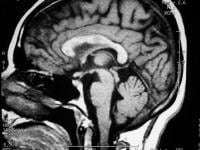 Sagittal T1-weighted MRI depicts multiple hypointense lesions in the corpus callosum; this finding is characteristic of multiple sclerosis.
Sagittal T1-weighted MRI depicts multiple hypointense lesions in the corpus callosum; this finding is characteristic of multiple sclerosis. 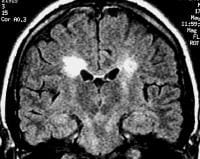 Coronal fluid-attenuated inversion recovery (FLAIR) MRI in a patient with multiple sclerosis demonstrates periventricular high–signal intensity lesions, which exhibit a typical distribution for multiple sclerosis. FLAIR MRI is a highly sensitive sequence for lesion detection, particularly supratentorially.
Coronal fluid-attenuated inversion recovery (FLAIR) MRI in a patient with multiple sclerosis demonstrates periventricular high–signal intensity lesions, which exhibit a typical distribution for multiple sclerosis. FLAIR MRI is a highly sensitive sequence for lesion detection, particularly supratentorially. 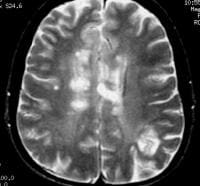 Axial T2-weighted MRI in a patient with multiple sclerosis demonstrates numerous white matter plaques in a callosal and pericallosal white matter distribution.
Axial T2-weighted MRI in a patient with multiple sclerosis demonstrates numerous white matter plaques in a callosal and pericallosal white matter distribution. 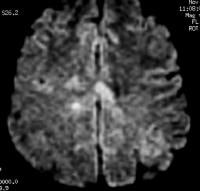 Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity. One of the limitations of using MRI in patients with MS is the discordance occurring between lesion location and the clinical presentation. In addition, depending on the number and location of findings, MRI can vary greatly in terms of sensitivity and specificity in the diagnosis of MS. This is especially true of primary progressive MS, which may not show the classic discrete lesions of relapsing-remitting MS.
Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity. One of the limitations of using MRI in patients with MS is the discordance occurring between lesion location and the clinical presentation. In addition, depending on the number and location of findings, MRI can vary greatly in terms of sensitivity and specificity in the diagnosis of MS. This is especially true of primary progressive MS, which may not show the classic discrete lesions of relapsing-remitting MS.
A clinician presented with an MRI report that details a few "nonspecific white matter lesions" that are "compatible with MS" is often frustrated with the lack of sensitivity and specificity of such a description. For this reason, imaging findings need to be described in detail, and preferably referenced to one of the published set of diagnostic criteria such as those by Paty[1] or Barkhof.[2] Finally, the specific patient's neurologic history and clinical findings must be correlated with the imaging to establish an accurate diagnosis.[3]
Plain radiographic studies have no positive predictive value in the diagnosis of multiple sclerosis, but occasionally, plain radiographs may be used to exclude mechanical bony lesions. Angiography also has a limited role in the diagnosis and management of MS, but when central nervous system (CNS) vasculitis is considered in a patient with undifferentiated findings, angiography may occasionally be considered.
Cerebrospinal fluid (CSF) analysis for oligoclonal banding or immunoglobulin G (IgG) levels is no longer routine in the investigation of MS, although this test may be of use when MRI is unavailable or MRI findings are nondiagnostic.[4]
Clinically, MS has historically been diagnosed via the demonstration of white matter dysfunction disseminated in time and space.[8] With the advent of diagnostic laboratory investigations and imaging techniques, the Poser criteria were proposed to establish a degree of certainty of diagnosis in the absence of the 2 clinical attacks by using terms such as possible MS and probable MS.[9]
With increasing treatment options for MS and better imaging techniques, newer diagnostic criteria have been suggested that allow diagnosis after a single attack coupled with appropriate positive test results. These criteria have been coined the MacDonald criteria.[10] Essentially, they allow for the second attack in time to be defined by a new lesion appearing on MRI. Also, the MacDonald criteria allow the dissemination in space to be established on the basis of either 9 typical white matter lesions on MRI or 1 enhancing lesion. If CSF studies show increased IgG values or oligoclonal banding, the presence of only 2 typical MRI lesions satisfy the dissemination-in-time criteria.
With respect to the initial clinical presentation in MS, it may vary with the white matter tract involved, and it may include somatic sensory changes, optic neuritis, or weakness, to mention just a few possible neurologic presentations. After only a single attack, the diagnosis of MS is suggested if the first impairment is coupled with positive paraclinical test results, such as those on imaging or CSF studies. Furthermore, the attack must be compatible with the pattern of impairment found in patients with MS, which typically means that the duration of deficit is days to weeks. Worsening of vision due to optic neuritis and subsequent exercise is known as the Uhthoff phenomenon.
Stankiewicz et al correlated brain lesions and clinical status with 1.5T and 3T MRI in 32 patients with MS by use of MRI fluid-attenuated inversion-recovery (FLAIR) sequences, and the authors found that MRI at 3T may provide increased sensitivity and validity in assessment of MS brain lesions. The study showed that FLAIR lesion volume (FLLV) at 3T was higher than at 1.5T. While 3T FLLV correlated moderately and significantly, 1.5T FLLV correlated poorly. When controlling for age and depression, correlations between FLLV and cognitive measures were significant at 1.5T for the Judgment of Line Orientation Test, the Symbol Digit Modalities Test, and the California Verbal Learning Test Delayed Free Recall, but at 3T, correlations were significant and of greater magnitude.[11]
Occasionally, the course of MS may be more indolent and exhibit a chronic, persistent neurologic deficit without apparent ongoing deterioration or further impairment. Sometimes, this course of MS is called inactive or benign MS, and this form is often observed in patients with prior relapsing-remitting disease.
Another potentially complicating matter clinically is that highly active MS lesions may sometimes demonstrate significant mass effect. Rarely, mass effect can lead to midline shift, herniations, infarctions, and even death. Such a drastic clinical and radiologic presentation can lead to an incorrect preliminary diagnosis and inappropriate neurosurgical intervention. When MS presents in a more fulminant, aggressive manner, it is frequently known as malignant MS or the Marburg variant.
In a prospective study, Lebrun et al followed 70 patients who had their first brain MRI for a variety of medical symptoms not suggestive of MS and found the mean time between the first brain MRI and the first clinically isolated syndrome to be 2.3 years (range, 0.8-5 y). Diagnostic studies of the blood, CSF, and visual evoked potentials were conducted, and clinical conversion occurred in 23 patients: 6 to optic neuritis, 6 to myelitis, 5 to brainstem symptoms, 4 to sensitive symptoms, 1 to cerebellar symptoms, and 1 to cognitive deterioration.[12] For patient education information, see Multiple Sclerosis.
Preferred examination
Radiologically, the use of MRI is revolutionizing the investigation, diagnosis, and even the treatment of MS (see the images below). Usually, MRI is the only imaging modality needed for imaging patients with MS, and it far surpasses all other tests with respect to its positive predictive value. Sagittal T1-weighted MRI depicts multiple hypointense lesions in the corpus callosum; this finding is characteristic of multiple sclerosis.
Sagittal T1-weighted MRI depicts multiple hypointense lesions in the corpus callosum; this finding is characteristic of multiple sclerosis.  Coronal fluid-attenuated inversion recovery (FLAIR) MRI in a patient with multiple sclerosis demonstrates periventricular high–signal intensity lesions, which exhibit a typical distribution for multiple sclerosis. FLAIR MRI is a highly sensitive sequence for lesion detection, particularly supratentorially.
Coronal fluid-attenuated inversion recovery (FLAIR) MRI in a patient with multiple sclerosis demonstrates periventricular high–signal intensity lesions, which exhibit a typical distribution for multiple sclerosis. FLAIR MRI is a highly sensitive sequence for lesion detection, particularly supratentorially.  Axial T2-weighted MRI in a patient with multiple sclerosis demonstrates numerous white matter plaques in a callosal and pericallosal white matter distribution.
Axial T2-weighted MRI in a patient with multiple sclerosis demonstrates numerous white matter plaques in a callosal and pericallosal white matter distribution.  Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity. One of the limitations of using MRI in patients with MS is the discordance occurring between lesion location and the clinical presentation. In addition, depending on the number and location of findings, MRI can vary greatly in terms of sensitivity and specificity in the diagnosis of MS. This is especially true of primary progressive MS, which may not show the classic discrete lesions of relapsing-remitting MS.
Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity. One of the limitations of using MRI in patients with MS is the discordance occurring between lesion location and the clinical presentation. In addition, depending on the number and location of findings, MRI can vary greatly in terms of sensitivity and specificity in the diagnosis of MS. This is especially true of primary progressive MS, which may not show the classic discrete lesions of relapsing-remitting MS.A clinician presented with an MRI report that details a few "nonspecific white matter lesions" that are "compatible with MS" is often frustrated with the lack of sensitivity and specificity of such a description. For this reason, imaging findings need to be described in detail, and preferably referenced to one of the published set of diagnostic criteria such as those by Paty[1] or Barkhof.[2] Finally, the specific patient's neurologic history and clinical findings must be correlated with the imaging to establish an accurate diagnosis.[3]
Plain radiographic studies have no positive predictive value in the diagnosis of multiple sclerosis, but occasionally, plain radiographs may be used to exclude mechanical bony lesions. Angiography also has a limited role in the diagnosis and management of MS, but when central nervous system (CNS) vasculitis is considered in a patient with undifferentiated findings, angiography may occasionally be considered.
Cerebrospinal fluid (CSF) analysis for oligoclonal banding or immunoglobulin G (IgG) levels is no longer routine in the investigation of MS, although this test may be of use when MRI is unavailable or MRI findings are nondiagnostic.[4]
Clinical diagnosis
A diagnosis of MS is made on the basis of clinical findings by using supporting evidence from ancillary tests such as CSF examination for oligoclonal banding and MRI.[5, 6, 7]Clinically, MS has historically been diagnosed via the demonstration of white matter dysfunction disseminated in time and space.[8] With the advent of diagnostic laboratory investigations and imaging techniques, the Poser criteria were proposed to establish a degree of certainty of diagnosis in the absence of the 2 clinical attacks by using terms such as possible MS and probable MS.[9]
With increasing treatment options for MS and better imaging techniques, newer diagnostic criteria have been suggested that allow diagnosis after a single attack coupled with appropriate positive test results. These criteria have been coined the MacDonald criteria.[10] Essentially, they allow for the second attack in time to be defined by a new lesion appearing on MRI. Also, the MacDonald criteria allow the dissemination in space to be established on the basis of either 9 typical white matter lesions on MRI or 1 enhancing lesion. If CSF studies show increased IgG values or oligoclonal banding, the presence of only 2 typical MRI lesions satisfy the dissemination-in-time criteria.
With respect to the initial clinical presentation in MS, it may vary with the white matter tract involved, and it may include somatic sensory changes, optic neuritis, or weakness, to mention just a few possible neurologic presentations. After only a single attack, the diagnosis of MS is suggested if the first impairment is coupled with positive paraclinical test results, such as those on imaging or CSF studies. Furthermore, the attack must be compatible with the pattern of impairment found in patients with MS, which typically means that the duration of deficit is days to weeks. Worsening of vision due to optic neuritis and subsequent exercise is known as the Uhthoff phenomenon.
Stankiewicz et al correlated brain lesions and clinical status with 1.5T and 3T MRI in 32 patients with MS by use of MRI fluid-attenuated inversion-recovery (FLAIR) sequences, and the authors found that MRI at 3T may provide increased sensitivity and validity in assessment of MS brain lesions. The study showed that FLAIR lesion volume (FLLV) at 3T was higher than at 1.5T. While 3T FLLV correlated moderately and significantly, 1.5T FLLV correlated poorly. When controlling for age and depression, correlations between FLLV and cognitive measures were significant at 1.5T for the Judgment of Line Orientation Test, the Symbol Digit Modalities Test, and the California Verbal Learning Test Delayed Free Recall, but at 3T, correlations were significant and of greater magnitude.[11]
Clinical course
The clinical course of MS can follow different patterns, and this observation has led to the classification of distinct types of MS. The most common form of MS is termed relapsing-remitting MS, in which progression involves symptoms of neurologic dysfunction frequently followed by partial or complete clinical recovery. In relapsing-remitting MS, global clinical deterioration has traditionally been attributed to cumulative deficit due to incomplete recovery from repeated occurrences of individual relapses. However, this cumulative deficit has been questioned, because evidence increasingly suggests an ongoing background neurologic deterioration that is independent of the relapses.Occasionally, the course of MS may be more indolent and exhibit a chronic, persistent neurologic deficit without apparent ongoing deterioration or further impairment. Sometimes, this course of MS is called inactive or benign MS, and this form is often observed in patients with prior relapsing-remitting disease.
Another potentially complicating matter clinically is that highly active MS lesions may sometimes demonstrate significant mass effect. Rarely, mass effect can lead to midline shift, herniations, infarctions, and even death. Such a drastic clinical and radiologic presentation can lead to an incorrect preliminary diagnosis and inappropriate neurosurgical intervention. When MS presents in a more fulminant, aggressive manner, it is frequently known as malignant MS or the Marburg variant.
In a prospective study, Lebrun et al followed 70 patients who had their first brain MRI for a variety of medical symptoms not suggestive of MS and found the mean time between the first brain MRI and the first clinically isolated syndrome to be 2.3 years (range, 0.8-5 y). Diagnostic studies of the blood, CSF, and visual evoked potentials were conducted, and clinical conversion occurred in 23 patients: 6 to optic neuritis, 6 to myelitis, 5 to brainstem symptoms, 4 to sensitive symptoms, 1 to cerebellar symptoms, and 1 to cognitive deterioration.[12] For patient education information, see Multiple Sclerosis.
The New York Review of Books: Someone Else's Children
To contact us Click HERE
The Burns Archive is pleased to announce our exhibition Reed Bontecou: Masterpieces of Civil War Portraiture and accompanying publication Shooting Soldiers have been covered by The New York Review of Books:

Someone Else’s Children
Christopher Benfey
November 28, 2011
My wife and I have two sons, aged eighteen and twenty-two. Both have registered for the Selective Service, as the law requires. (“Our objective is to register you,” the official letter reminded them, “not to have you prosecuted.”) We don’t have a clear idea of Tommy’s or Nicholas’s views regarding military service; we hope that circumstances won’t force us to find out. None of us knows any men or women currently serving in Iraq or Afghanistan. They are someone else’s children. We watch news reports of wounded veterans learning to walk with prosthetic limbs. Recent stories about body parts mislaid at the military mortuary at Dover Air Force Base fill us with outrage. Still, for many of us, it is a general, not an individualized outrage.
During the Civil War, in contrast, the mangling of young bodies was evident to all. Three million volunteers armed with advanced rifles, and firing at one another at point-blank range, fought on battlefields often not far from their own homes. American writers, many of whom had children in the war, were not insulated from the carnage. Fred Stowe was standing in the graveyard on Cemetery Ridge, above Gettysburg, when a live shell exploded near his ear, opening a wound that never healed. Charles Longfellow sought distraction from the trauma of the war in Yokohama, where he had a giant carp tattooed across his back, around the scars of two bullet holes. Emily Dickinson chose as her literary advisor a Union colonel suffering from PTSD: “We can find no scar,” she wrote in a famous poem, “But internal difference— / Where the Meanings, are.”
Louisa May Alcott and Walt Whitman served as nurses and eyewitness reporters in the hideous Union hospitals in Washington, D. C. Alcott contracted typhoid in the septic wards and wrote Little Women, about the daughters of a father wounded in the war, while treating herself with mercury. Whitman ministered to the needs of wounded soldiers while also keeping a careful visual record of everything he saw, “this other freight of helpless worn and wounded youth,” as he wrote to Emerson. “Doctors sawed arms & legs off from morning till night,” he reported in his journal. He was dismayed to see “a heap of feet, arms, legs, etc., under a tree in front of a hospital.” As he moved from bed to bed in the overcrowded wards, he was shocked by the youth of the victims. “Charles Miller, bed 19, company D, 53rd Pennsylvania, is only sixteen years of age, very bright, courageous boy, left leg amputated below the knee.”
The remarkable medical photographs of the Civil War surgeon-photographer Reed Bontecou—now published in their entirety for the first time and recently shown at The Robert Anderson gallery in New York—bring us closer still. Bontecou, from Troy, New York, was a classifier of seashells and an ornithologist who had traveled in the Amazon before the war collecting specimens. A pioneer in surgical procedures known for the dexterity and speed of his operations, he was also a photographer of genius. His iconic image, “A Morning’s Work,” shows a pile of amputated legs he himself had sawed off earlier that day. Bontecou’s albums served many ends, most obviously instruction, with before-and-after shots, in the identification and treatment of conditions like gangrene and bullets lodged in bone. But they also aided in the later identification of veterans for disbursement of disability and pension funds. Bontecou was apparently an engaging and capable administrator of army hospitals who was once threatened with disciplinary action for inviting a recovering Confederate officer to his home for Thanksgiving dinner.
Most poignant and painful is Bontecou’s artistic ability to capture the terror of his patients, what the editor and collector of medical photographs Stanley Burns, M.D., calls “individual bereavement.” Pvt. John Parmenter, unbearably young, lies prone on an army cot with his beautiful and vulnerable face turned towards us and his gangrenous foot propped up on a cushion. Then, in another photograph, we see him lying deathly pale and unconscious; a surgeon with his hand on one of Parmenter’s bent knees looks down thoughtfully at the severed foot. The picture has some of the bleak, geometrical power of Jacques-Louis David’s Death of Marat.
In another arresting image, Robert Fryer, eighteen years old and wearing his cap and uniform, all gold buttons carefully buttoned, holds his hand to his chest as though playfully mimicking a handgun. His features are deadpan. At first, we assume his hand is partially hidden in his jacket. But no, it’s an illusion, presumably deliberate on the part of the photographer. Fryer’s middle, ring, and little fingers are amputated. According to Bontecou’s notes, “Patient has good use of forefinger and thumb.” Perhaps he watched young Robert Fryer buttoning his coat.
The photographs are a bitter reminder of the hideous race between better medical response and ever more devastating weaponry. If, as Burns notes, the improvised explosive device (IED) has changed the way war is fought and the wounded treated today, the novelty of the Crimean War and the Civil War was the 58 caliber Minie Ball, named for its inventor, Claude-Etienne Minié. This was a war in which 94% of Union wounds were caused by bullets. The Minie Ball, Burns remarks, “shattered and fractured bone easily and commonly carried clothing and other debris with it into the wound, making infection a constant companion in almost every case.” Bontecou’s images “documented the battle against gunshot wounds,” at a time when battle armor was minimal or absent and two years before the discovery of the principles of antiseptic surgery in 1867. Burns adds grimly, “Many of the men we see here are going to die.”
There is another race on display in these photographs, between the sheer horror of the army hospital and our ability to find words and images adequate to the horror. “The real war,” Whitman wrote in Specimen Days, “will never get in the books.” The simple identification boards that many of Bontecou’s patients hold in their hands, with their name and company inscribed in white chalk, carry their own dire and individualized lyricism, as though to say, in Whitman’s resonant words: “I am the man, I suffered, I was there.” Andsell H. Beam, shot in the skull on April 6th, 1865, bows over his identification board as though in prayer, or in simple disbelief in his unfathomable fate. “Now that I have lived for 8 or 9 days amid such scenes as the camps furnish,” Whitman wrote his mother, “… really nothing we call trouble seems worth talking about.”
Shooting Soldiers: Civil War Medical Photography By R.B. Bontecou by Dr. Stanley M. Burns has recently been published.

Someone Else’s Children
Christopher Benfey
November 28, 2011
My wife and I have two sons, aged eighteen and twenty-two. Both have registered for the Selective Service, as the law requires. (“Our objective is to register you,” the official letter reminded them, “not to have you prosecuted.”) We don’t have a clear idea of Tommy’s or Nicholas’s views regarding military service; we hope that circumstances won’t force us to find out. None of us knows any men or women currently serving in Iraq or Afghanistan. They are someone else’s children. We watch news reports of wounded veterans learning to walk with prosthetic limbs. Recent stories about body parts mislaid at the military mortuary at Dover Air Force Base fill us with outrage. Still, for many of us, it is a general, not an individualized outrage.
 |
| R.B. Bontencou, Courtesey of Stanley B. Burns, MD Charles H. Greenfield (left), wounded April 2, 1865 at Petersberg, VA; A. Smith (center), wounded April 16, 1864 at Southside Railroad; P. Ferris (right), gunshot wound, left leg. |
During the Civil War, in contrast, the mangling of young bodies was evident to all. Three million volunteers armed with advanced rifles, and firing at one another at point-blank range, fought on battlefields often not far from their own homes. American writers, many of whom had children in the war, were not insulated from the carnage. Fred Stowe was standing in the graveyard on Cemetery Ridge, above Gettysburg, when a live shell exploded near his ear, opening a wound that never healed. Charles Longfellow sought distraction from the trauma of the war in Yokohama, where he had a giant carp tattooed across his back, around the scars of two bullet holes. Emily Dickinson chose as her literary advisor a Union colonel suffering from PTSD: “We can find no scar,” she wrote in a famous poem, “But internal difference— / Where the Meanings, are.”
 |
| R.B. Bontencou, Courtesey of Stanley B. Burns, MD “A Morning’s Work” R. B. Bontecou’s label for his iconic image of wartime labors, 1865. |
Louisa May Alcott and Walt Whitman served as nurses and eyewitness reporters in the hideous Union hospitals in Washington, D. C. Alcott contracted typhoid in the septic wards and wrote Little Women, about the daughters of a father wounded in the war, while treating herself with mercury. Whitman ministered to the needs of wounded soldiers while also keeping a careful visual record of everything he saw, “this other freight of helpless worn and wounded youth,” as he wrote to Emerson. “Doctors sawed arms & legs off from morning till night,” he reported in his journal. He was dismayed to see “a heap of feet, arms, legs, etc., under a tree in front of a hospital.” As he moved from bed to bed in the overcrowded wards, he was shocked by the youth of the victims. “Charles Miller, bed 19, company D, 53rd Pennsylvania, is only sixteen years of age, very bright, courageous boy, left leg amputated below the knee.”
 |
| R.B. Bontencou, Courtesey of Stanley B. Burns, MD Pvt. John Parmenter, before being operated on for gangrene. Wounded at Amelia Springs, VA, April 3, 1865 |
The remarkable medical photographs of the Civil War surgeon-photographer Reed Bontecou—now published in their entirety for the first time and recently shown at The Robert Anderson gallery in New York—bring us closer still. Bontecou, from Troy, New York, was a classifier of seashells and an ornithologist who had traveled in the Amazon before the war collecting specimens. A pioneer in surgical procedures known for the dexterity and speed of his operations, he was also a photographer of genius. His iconic image, “A Morning’s Work,” shows a pile of amputated legs he himself had sawed off earlier that day. Bontecou’s albums served many ends, most obviously instruction, with before-and-after shots, in the identification and treatment of conditions like gangrene and bullets lodged in bone. But they also aided in the later identification of veterans for disbursement of disability and pension funds. Bontecou was apparently an engaging and capable administrator of army hospitals who was once threatened with disciplinary action for inviting a recovering Confederate officer to his home for Thanksgiving dinner.
 |
| R.B. Bontencou, Courtesey of Stanley B. Burns, MD Pvt. John Parmenter lying unconscious from anesthesia on operating table with his severed foot, 1865. |
Most poignant and painful is Bontecou’s artistic ability to capture the terror of his patients, what the editor and collector of medical photographs Stanley Burns, M.D., calls “individual bereavement.” Pvt. John Parmenter, unbearably young, lies prone on an army cot with his beautiful and vulnerable face turned towards us and his gangrenous foot propped up on a cushion. Then, in another photograph, we see him lying deathly pale and unconscious; a surgeon with his hand on one of Parmenter’s bent knees looks down thoughtfully at the severed foot. The picture has some of the bleak, geometrical power of Jacques-Louis David’s Death of Marat.
 |
| R.B. Bontencou, Courtesey of Stanley B. Burns, MD Robert Fryer, Private, Age 18. Gunshot wound, right hand. Wounded March 25, 1865, at Battle of Hatcher’s Run. |
The photographs are a bitter reminder of the hideous race between better medical response and ever more devastating weaponry. If, as Burns notes, the improvised explosive device (IED) has changed the way war is fought and the wounded treated today, the novelty of the Crimean War and the Civil War was the 58 caliber Minie Ball, named for its inventor, Claude-Etienne Minié. This was a war in which 94% of Union wounds were caused by bullets. The Minie Ball, Burns remarks, “shattered and fractured bone easily and commonly carried clothing and other debris with it into the wound, making infection a constant companion in almost every case.” Bontecou’s images “documented the battle against gunshot wounds,” at a time when battle armor was minimal or absent and two years before the discovery of the principles of antiseptic surgery in 1867. Burns adds grimly, “Many of the men we see here are going to die.”
 |
| R.B. Bontencou, Courtesey of Stanley B. Burns, MD Andsell H. Beam, Age 24. Gunshot wound to head. Wounded April 6, 1865, Battle Of Farmville, VA. |
Shooting Soldiers: Civil War Medical Photography By R.B. Bontecou by Dr. Stanley M. Burns has recently been published.
24 Haziran 2012 Pazar
I'm back, with good news
To contact us Click HERE
I know I've been gone a while. I won't count off the months and days.
My father-in-law passed away after my last post. Cancer is a terrible thing. It was his second bout with the disease and, sadly, he went undiagnosed until Stage IV the second time around. Chemotherapy gave him some extra time but it didn't seem to be enough. I really miss Bill, and I always will.
On the way home from his funeral ceremony, which was delayed a few weeks for the Christmas holidays, I got the news that my mother was taking a turn for the worse. I flew out and drove straight to her beside getting there just hours before she drifted off into a coma. I never did get to talk to her in any meaningful sense, but that has defined our relationship for 36 years. I think its more difficult to lose a parent you weren't close to sometimes, at least initially. I spent the rest of my trip fighting an adrenal crisis that wouldn't let go.
Meanwhile the surgeon that will be performing my pituitary procedure insisted that I undergo an Inferior Petrosal Sinus Sampling. That's fine, as it assists him when mapping out an approach for surgery. However, arranging for the IPSS was another story. It took from the beginning of January until March 28th to have it scheduled, confirmed and carried out. Then another three weeks for informal results, four weeks for the official ones with a report.
I am happy to say that I do have a date for surgery: May 24th and I do have hope that this Christmas I'll feel better.
I seem to tick off years at Christmas, thinking 'Last year I thought I'd be better by now'. Maybe this is my year.
My father-in-law passed away after my last post. Cancer is a terrible thing. It was his second bout with the disease and, sadly, he went undiagnosed until Stage IV the second time around. Chemotherapy gave him some extra time but it didn't seem to be enough. I really miss Bill, and I always will.
On the way home from his funeral ceremony, which was delayed a few weeks for the Christmas holidays, I got the news that my mother was taking a turn for the worse. I flew out and drove straight to her beside getting there just hours before she drifted off into a coma. I never did get to talk to her in any meaningful sense, but that has defined our relationship for 36 years. I think its more difficult to lose a parent you weren't close to sometimes, at least initially. I spent the rest of my trip fighting an adrenal crisis that wouldn't let go.
Meanwhile the surgeon that will be performing my pituitary procedure insisted that I undergo an Inferior Petrosal Sinus Sampling. That's fine, as it assists him when mapping out an approach for surgery. However, arranging for the IPSS was another story. It took from the beginning of January until March 28th to have it scheduled, confirmed and carried out. Then another three weeks for informal results, four weeks for the official ones with a report.
I am happy to say that I do have a date for surgery: May 24th and I do have hope that this Christmas I'll feel better.
I seem to tick off years at Christmas, thinking 'Last year I thought I'd be better by now'. Maybe this is my year.
Damn you, Cushing's Disease
To contact us Click HERE
How to disappoint all of your friends, relatives and two young children: Spend a month recuperating from pituitary surgery in June - and not have your remission stick. In September I realized that the Cushing's had returned, if it ever left, and I'm just now ready to admit to it.
Thankfully the road is paved already and my specialist is recommending a repeat surgery. Tomorrow I go in for a pituitary MRI and with any luck we can find the source. The abdominal MRI from two weeks ago shows normal adrenal glands. In a twisted way I was hoping that my adrenals were the culprit, even though it goes against all likelihood. Removing the adrenal glands would almost certainly put an end to the Cushing's misery. I'd take Addison's back with a warm heart, since my weight has ballooned beyond all recognition. (I avoid mirrors.) They discovered a cyst on my spleen and liver during the abdominal MRI. No one has addressed those findings, so I don't know what to make of it. A quick Google tells me that it is rare, ha - who knew?, and LORD HELP ME, I now know what 'non-parasitic' means. *la la la la la la* (fingers in ears)
I have some more blood testing to do at midnight, which runs a close second to my most despised form of testing torture, and the MRI tomorrow. As far as symptoms go, weight gain - cystic 'steroid' acne - flank pain - and very recently a deterioration of the vision in my left eye (again). The double vision is slight and I only get migraines once a week or so. My bout with constant 24 hour a day headache & migraine has ruined my ability to describe pain. I cannot fathom calling the first surgery a 'failure', since it single-handedly took away the constant headache.
I'm rambling and it is late.... I'll be back with MRI results soon.
Thankfully the road is paved already and my specialist is recommending a repeat surgery. Tomorrow I go in for a pituitary MRI and with any luck we can find the source. The abdominal MRI from two weeks ago shows normal adrenal glands. In a twisted way I was hoping that my adrenals were the culprit, even though it goes against all likelihood. Removing the adrenal glands would almost certainly put an end to the Cushing's misery. I'd take Addison's back with a warm heart, since my weight has ballooned beyond all recognition. (I avoid mirrors.) They discovered a cyst on my spleen and liver during the abdominal MRI. No one has addressed those findings, so I don't know what to make of it. A quick Google tells me that it is rare, ha - who knew?, and LORD HELP ME, I now know what 'non-parasitic' means. *la la la la la la* (fingers in ears)
I have some more blood testing to do at midnight, which runs a close second to my most despised form of testing torture, and the MRI tomorrow. As far as symptoms go, weight gain - cystic 'steroid' acne - flank pain - and very recently a deterioration of the vision in my left eye (again). The double vision is slight and I only get migraines once a week or so. My bout with constant 24 hour a day headache & migraine has ruined my ability to describe pain. I cannot fathom calling the first surgery a 'failure', since it single-handedly took away the constant headache.
I'm rambling and it is late.... I'll be back with MRI results soon.
I've had it. Up to my neck, err - brain.
To contact us Click HERE
My brain is still leaking. Dammit.
"My brain is leaking! My brain is leaking!"
Friday will mark four weeks since surgery and I am spitting mad that they didn't believe me when I told them I had a leak at the hospital. Sorry, dear city of Houston, I don't think we have a future together. We don't seem to mesh well. ;)
I've tried laying flat on my back every night when I sleep and it isn't helping. Can I just say I deserve a medal for even sleeping like that for one night, let alone four or five? Yes, I do. I've had to resort to sleeping on the couch so that the width of sleeping area discourages my sleeping self from turning.
I'm trying to arrange for the radiological tests to determine the exact location of the drip, and have an appointment with an ENT/surgeon on Monday. There is no way I am going under without them knowing exactly where to stick in a plug. *sigh*
I started the daily growth hormone shots seven days ago. It is really starting to help. Now I can almost actually lift my feet when I walk. Yeehaw!
I'm making an effort to get off of the steroids so that I can start testing again soon. I managed to drop from 60mg of cortef to 20mg in less than five days. *blowing on knuckles, rubbing on chest* Holy wean, Batman! :)
"My brain is leaking! My brain is leaking!"

Friday will mark four weeks since surgery and I am spitting mad that they didn't believe me when I told them I had a leak at the hospital. Sorry, dear city of Houston, I don't think we have a future together. We don't seem to mesh well. ;)
I've tried laying flat on my back every night when I sleep and it isn't helping. Can I just say I deserve a medal for even sleeping like that for one night, let alone four or five? Yes, I do. I've had to resort to sleeping on the couch so that the width of sleeping area discourages my sleeping self from turning.
I'm trying to arrange for the radiological tests to determine the exact location of the drip, and have an appointment with an ENT/surgeon on Monday. There is no way I am going under without them knowing exactly where to stick in a plug. *sigh*
I started the daily growth hormone shots seven days ago. It is really starting to help. Now I can almost actually lift my feet when I walk. Yeehaw!
I'm making an effort to get off of the steroids so that I can start testing again soon. I managed to drop from 60mg of cortef to 20mg in less than five days. *blowing on knuckles, rubbing on chest* Holy wean, Batman! :)
I'm a big weaner
To contact us Click HERE
I've managed to wean from 60mg of hydrocortisone to 5mg in less than 7 days! Yay! Yippee. Actually I think I could have skipped the 5mg today, but I took it just for the hell of it. One tiny ping in my adrenals and I dropped 5mg, you know, just in case.
Ok, honestly? That isn't good news in the Cushing's world. My body shouldn't be able to compensate for that rapid of a drop in steroids in such a short time. I suppose it means that the source of my Cushing's is finding a way to come back to life. *fingers in ears* LA LA LA LA LA
I saw my primary doctor's assistant today to see if they could order a radiological test to find the source of my leaky brain. They were pretty stumped. I don't want to bug them, but they haven't called me yet. They said they would. I'm afraid he'll drop me as a patient if I keep 'coming down' with questions and problems that they can't deal with. I really like him and his staff; I try to send them goodies for the office whenever they help me through situations like the one I am dealing with. He must really like people with strep or UTIs after my visits. ;)
I made an appointment with an ENT surgeon for Monday, just to cover my bases. Once again, I hope I don't scare the bejeezus out of him and have him send me packing. It was already suggested that I go back to Houston for the leak repair. No. Freaking. Way. is my non-verbalized response.
I know a neurosurgeon in Los Angeles, but I don't think a neurosurgeon is needed for this - more an ENT problem. I think. I don't know. Which brings me to my most recent thought: wouldn't it be nice to just have NORMAL PROBLEMS? How about that osteoarthritis I have in all of my left-side joints. Couldn't that be my only cross to bear? Wouldn't that be nice?!? I try not to talk about my medical issues out loud to normal people any more. I scare them. *ducking*
Ok, honestly? That isn't good news in the Cushing's world. My body shouldn't be able to compensate for that rapid of a drop in steroids in such a short time. I suppose it means that the source of my Cushing's is finding a way to come back to life. *fingers in ears* LA LA LA LA LA
I saw my primary doctor's assistant today to see if they could order a radiological test to find the source of my leaky brain. They were pretty stumped. I don't want to bug them, but they haven't called me yet. They said they would. I'm afraid he'll drop me as a patient if I keep 'coming down' with questions and problems that they can't deal with. I really like him and his staff; I try to send them goodies for the office whenever they help me through situations like the one I am dealing with. He must really like people with strep or UTIs after my visits. ;)
I made an appointment with an ENT surgeon for Monday, just to cover my bases. Once again, I hope I don't scare the bejeezus out of him and have him send me packing. It was already suggested that I go back to Houston for the leak repair. No. Freaking. Way. is my non-verbalized response.
I know a neurosurgeon in Los Angeles, but I don't think a neurosurgeon is needed for this - more an ENT problem. I think. I don't know. Which brings me to my most recent thought: wouldn't it be nice to just have NORMAL PROBLEMS? How about that osteoarthritis I have in all of my left-side joints. Couldn't that be my only cross to bear? Wouldn't that be nice?!? I try not to talk about my medical issues out loud to normal people any more. I scare them. *ducking*
Happy National Cushing's Disease Awareness Day
To contact us Click HERE
Today is National Cushing's Awareness Day.
I asked a few local papers (ok, is LA considered "local"? ) to do an article about Cushing's. Long story short, only one Cushing's article in the entire USA, that I can find.
Regardless, in doing my search I found an article about a new veterinarian in-house lab machine that will do endocrinology tests while the patients wait.
MEANWHILE, I had my post-op gallon of blood drawn on Saturday. It is Tuesday and I am checking my online fax account for the results umpteen times a day.
Allow me to repeat this, because I do not have enough swear words in my vocabulary:
VETERINARIAN IN-HOUSE LAB EQUIPMENT - PRODUCING RESULTS FOR ANIMALS AND THEIR OWNERS WHILE THEY WAIT
I give up. I give up. I give up.
I'm going to schedule an appointment with a veterinarian. Canine cushing's disease: They get all the press, no one doubts them and now they can get their results in the office. WTF???
I asked a few local papers (ok, is LA considered "local"? ) to do an article about Cushing's. Long story short, only one Cushing's article in the entire USA, that I can find.
Regardless, in doing my search I found an article about a new veterinarian in-house lab machine that will do endocrinology tests while the patients wait.
MEANWHILE, I had my post-op gallon of blood drawn on Saturday. It is Tuesday and I am checking my online fax account for the results umpteen times a day.
Allow me to repeat this, because I do not have enough swear words in my vocabulary:
VETERINARIAN IN-HOUSE LAB EQUIPMENT - PRODUCING RESULTS FOR ANIMALS AND THEIR OWNERS WHILE THEY WAIT
I give up. I give up. I give up.
I'm going to schedule an appointment with a veterinarian. Canine cushing's disease: They get all the press, no one doubts them and now they can get their results in the office. WTF???
23 Haziran 2012 Cumartesi
Brain Imaging in Multiple Sclerosis _Computed Tomography
To contact us Click HERE
Similar to radiography, computed tomography (CT) scanning has had a limited role in the diagnosis of MS and in the treatment of patients since the advent of MRI. CT scans may be used to exclude other causes of neurologic impairment, but they have a low positive predictive value in the diagnosis of MS; thus, the false-negative rate is high.
Prior to the use of MRI, CT scanning, with the injection of double or triple doses of intravenous contrast material, was used in attempts to identify active MS lesions. However, the scans were insensitive for the detection of chronic lesions. CT scans can help in assessing the degree of cerebral atrophy associated with advanced MS, but given the plethora of additional information provided by MRI, CT is no longer used for this purpose.
An acute MS lesion may enhance and appear simply as an enhancing white matter lesion on CT scans, but the appearance is highly nonspecific. When a highly active MS lesion is observed to enhance and possibly exerts mass effect, it can be termed tumefactive (due to the potential for misidentification as a tumor). Because CT scans typically do not help to identify the more chronic lesions, the tumefactive MS lesion may appear as a solitary enhancing mass, which leads to neurosurgical intervention. Fortunately, this situation is relatively uncommon.
In a cohort of 200 patients, Paty et al found that of the 19 who went on to develop clinically definite MS (CDMS), abnormal CT findings were demonstrated in only 9 (47%). In contrast, abnormal MRI findings were demonstrated in 18 (95%). All of the abnormal CT findings were also demonstrated on MRIs.
Prior to the use of MRI, CT scanning, with the injection of double or triple doses of intravenous contrast material, was used in attempts to identify active MS lesions. However, the scans were insensitive for the detection of chronic lesions. CT scans can help in assessing the degree of cerebral atrophy associated with advanced MS, but given the plethora of additional information provided by MRI, CT is no longer used for this purpose.
An acute MS lesion may enhance and appear simply as an enhancing white matter lesion on CT scans, but the appearance is highly nonspecific. When a highly active MS lesion is observed to enhance and possibly exerts mass effect, it can be termed tumefactive (due to the potential for misidentification as a tumor). Because CT scans typically do not help to identify the more chronic lesions, the tumefactive MS lesion may appear as a solitary enhancing mass, which leads to neurosurgical intervention. Fortunately, this situation is relatively uncommon.
In a cohort of 200 patients, Paty et al found that of the 19 who went on to develop clinically definite MS (CDMS), abnormal CT findings were demonstrated in only 9 (47%). In contrast, abnormal MRI findings were demonstrated in 18 (95%). All of the abnormal CT findings were also demonstrated on MRIs.
Brain Imaging in Multiple Sclerosis _Magnetic Resonance Imaging
To contact us Click HERE
The advent of MRI has revolutionized the diagnosis and monitoring of MS. MRI is well established as the preferred imaging modality for depicting MS lesions. In patients with clinically definite MS (CDMS), MRI demonstrates a high rate of abnormal findings compatible with the diagnosis. In a study by Lukes et al, lesions were demonstrated in 10 patients with CDMS.[13] In a larger study by Robertson et al, MRI findings were abnormal in 124 of 133 patients with CDMS. Ormerod et al found that 112 of 114 patients with CDMS had abnormal MRI findings and that 102 of 114 had discrete white matter lesions.[14]
Another major use of MRI has been the evaluation of patients who have had only 1 episode of neurologic impairment and who do not meet the clinical criteria for the diagnosis. The overall risk of developing MS after a single episode of neurologic impairment is estimated to be as low as 12% (2y follow-up study by Beck et al) to as high as 45% (12.9y follow-up study by Sandberg-Wollheim et al[15] ) or 58% (14.9y follow-up study by Rizzo et al[16] ).
MRI has been proven to be the most useful investigation for predicting the progression to MS. In a 10-year follow-up study of patients with a clinically isolated event, 45 (83%) of 54 patients with abnormal MRI findings went on to develop clinical MS, whereas only 3 of 27 with normal MRI findings developed MS.[17]
The criteria of Fazekas et al resulted in the same sensitivity and specificity. These criteria require 3 lesions with 2 of the 3 following characteristics: infratentorial location, periventricular location, and lesion greater than 6mm. The criteria of Barkhof require 1 infratentorial lesion, 1 juxtacortical lesion, 3 periventricular lesions, and either 1 gadolinium-enhanced lesion or more than 9 lesions on T2-weighted MRI scans. These criteria resulted in a sensitivity of 73% and a specificity of 73%. Thus, as the MRI criteria become more stringent in the diagnosis of MS, specificity increases at the expense of decreasing sensitivity.
In a cohort of the BENEFIT study (a multicenter, randomized, clinical study of 468 patients), the modified Barkhof criteria showed moderate predictive value for conversion to CDMS over 3 years, despite the fact that all patients received interferon beta-1b therapy for at least 1 year. Follow-up MRI was found to be most informative after 9 months in patients without dissemination in space at baseline. The overall conversion rate to CDMS was 42%. Barkhof criteria with the strongest prognostic value were the presence at baseline of at least 9 T2-weighted lesions and at least 3 periventricular lesions.[20]
According to a study of postmortem MS tissue by Pitt et al, 3-dimensional (3-D), T2*-weighted, gradient-echo (T2*GRE) and white matter–attenuated, turbo-field-echo (TFE) sequences at a 7T field strength can detect most cortical lesions. The 3-D T2*GRE and white matter–attenuated TFE sequences retrospectively detected 93% and 82% of all cortical lesions, respectively.[21]
Lesions may be observed anywhere in the CNS white matter, including the supratentorium, infratentorium, and spinal cord; however, more typical locations for MS lesions include the periventricular white matter, brainstem, cerebellum, and spinal cord. Ovoid lesions perpendicular to the ventricles are common in MS and occasionally are called Dawson bars or fingers, which occur along the path of the deep medullary veins. Perhaps the most specific lesions in MS are noted in the corpus callosum at the interface with the septum pellucidum.[27] The imaging characteristics of MS are depicted on the MRI scans below.
 Sagittal T1-weighted MRI depicts multiple hypointense lesions in the corpus callosum; this finding is characteristic of multiple sclerosis.
Sagittal T1-weighted MRI depicts multiple hypointense lesions in the corpus callosum; this finding is characteristic of multiple sclerosis.  Axial T2-weighted MRI in a patient with multiple sclerosis demonstrates numerous white matter plaques in a callosal and pericallosal white matter distribution.
Axial T2-weighted MRI in a patient with multiple sclerosis demonstrates numerous white matter plaques in a callosal and pericallosal white matter distribution. 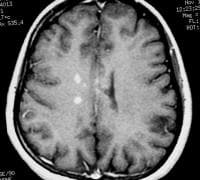 Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis demonstrates several intensely enhancing pericallosal white matter lesions compatible with active disease.
Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis demonstrates several intensely enhancing pericallosal white matter lesions compatible with active disease. 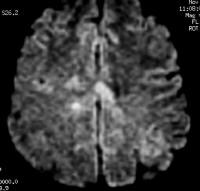 Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity.
Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity. 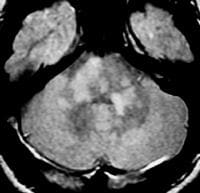 Axial proton density–weighted MRI through the posterior fossa in a patient with multiple sclerosis demonstrates multiple bright foci in the brainstem and cerebellum. Proton density–weighted sequences are highly sensitive for the detection of plaques in multiple sclerosis, especially in the posterior fossa.
Axial proton density–weighted MRI through the posterior fossa in a patient with multiple sclerosis demonstrates multiple bright foci in the brainstem and cerebellum. Proton density–weighted sequences are highly sensitive for the detection of plaques in multiple sclerosis, especially in the posterior fossa. 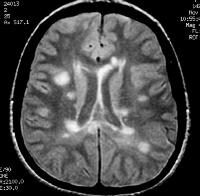 Axial proton density–weighted MRI demonstrates multiple lesions in a distribution characteristic of multiple sclerosis. Specifically, the periventricular lesions and the more peripheral white matter lesions near the gray matter–white matter junction are typical MRI findings in multiple sclerosis.
Axial proton density–weighted MRI demonstrates multiple lesions in a distribution characteristic of multiple sclerosis. Specifically, the periventricular lesions and the more peripheral white matter lesions near the gray matter–white matter junction are typical MRI findings in multiple sclerosis. 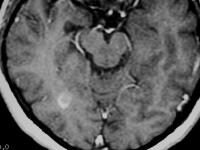 Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis depicts enhancement of a plaque in the right temporo-occipital lobe, signifying disease activity. Note the C-shaped, or arclike, enhancement, which is fairly characteristic of multiple sclerosis.
Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis depicts enhancement of a plaque in the right temporo-occipital lobe, signifying disease activity. Note the C-shaped, or arclike, enhancement, which is fairly characteristic of multiple sclerosis. 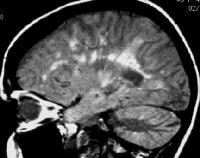 Sagittal proton density–weighted MRI in a patient with multiple sclerosis demonstrates the characteristic corpus callosal and pericallosal white matter lesions.
Sagittal proton density–weighted MRI in a patient with multiple sclerosis demonstrates the characteristic corpus callosal and pericallosal white matter lesions. 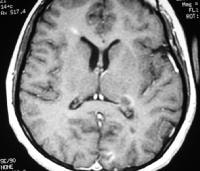 Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis depicts several enhancing lesions, at least 2 of which show characteristic C-shaped, or arclike, peripheral enhancement.
Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis depicts several enhancing lesions, at least 2 of which show characteristic C-shaped, or arclike, peripheral enhancement.  Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity. Proton density (PD)–weighted MRI has an advantage over standard T2 imaging, because on PD series, MS lesions remain hyperintense, while the CSF signal is suppressed. Therefore, the lesions are easily identified. Depending on the PD technique, the CSF signal is suppressed to a variable degree, rendering it isointense to hypointense relative to the brain parenchyma. This sequence results in substantial suppression of Virchow-Robin spaces, which are perivascular CSF spaces that may penetrate to the subcortical white matter. These spaces may appear as hyperintense spots on standard T2-weighted MRI scans.
Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity. Proton density (PD)–weighted MRI has an advantage over standard T2 imaging, because on PD series, MS lesions remain hyperintense, while the CSF signal is suppressed. Therefore, the lesions are easily identified. Depending on the PD technique, the CSF signal is suppressed to a variable degree, rendering it isointense to hypointense relative to the brain parenchyma. This sequence results in substantial suppression of Virchow-Robin spaces, which are perivascular CSF spaces that may penetrate to the subcortical white matter. These spaces may appear as hyperintense spots on standard T2-weighted MRI scans.
Compared with other techniques, nonenhanced T1-weighted MRI is far less sensitive in detecting MS lesions. Acute lesions usually are not depicted at all. With T1-weighted MRI, the clinician can gain a general appreciation of the global cerebral atrophy that occurs with advanced chronic MS. Global atrophy has been suggested to have the strongest imaging correlation with disability.
Chronic MS lesions usually result in localized leukomalacia, and they may appear as hypointense lesions that represent loss of tissue.
Gadolinium-enhanced T1-weighted MRI scans can depict acute, active MS lesions. These appear as enhancing white matter lesions; the presence of an enhancing lesion has been shown to increase the specificity for MS.[2, 18]
FLAIR MRI is a heavily T2-weighted technique that dampens the ventricular (ie, free-water) CSF signal. Thus, the highest signals on the sequence are from certain brain parenchymal abnormalities, such as MS lesions, while the CSF appears black. This appearance is different from that on PD-weighted MRIs, on which periventricular MS lesions may appear nearly isointense to the adjacent CSF. (See the image below.)
 Coronal fluid-attenuated inversion recovery (FLAIR) MRI in a patient with multiple sclerosis demonstrates periventricular high–signal intensity lesions, which exhibit a typical distribution for multiple sclerosis. FLAIR MRI is a highly sensitive sequence for lesion detection, particularly supratentorially. The greater relative suppression of CSF on FLAIR images compared with PD-weighted series increases the contrast between periventricular lesions and CSF, enhancing their detection. FLAIR has been shown to be superior to PD-weighted sequences in the detection of MS lesions in the cerebral hemispheres. However, PD-weighted imaging remains the investigation of choice for infratentorial lesions.[28]
Coronal fluid-attenuated inversion recovery (FLAIR) MRI in a patient with multiple sclerosis demonstrates periventricular high–signal intensity lesions, which exhibit a typical distribution for multiple sclerosis. FLAIR MRI is a highly sensitive sequence for lesion detection, particularly supratentorially. The greater relative suppression of CSF on FLAIR images compared with PD-weighted series increases the contrast between periventricular lesions and CSF, enhancing their detection. FLAIR has been shown to be superior to PD-weighted sequences in the detection of MS lesions in the cerebral hemispheres. However, PD-weighted imaging remains the investigation of choice for infratentorial lesions.[28]
Arnold et al noted that the NAA-Cr ratio in the CNS was decreased in moderate to advanced MS. White matter that appeared normal on T1- and T2-weighted images also demonstrated the reduction.[29] In addition, a normal ratio was noted in the area of a recently active lesion associated with clinical deficits that subsequently resolved. The findings led the authors to propose that MR spectroscopic findings may be able to help identify irreversible axonal damage.
In a study involving 88 patients with MS, De Stefano et al found a strong correlation between disability scores and NAA-Cr ratios.[30] The ratio exhibited a stronger correlation in patients with MS patients who had milder disability scores. Because MR spectroscopy appears to be capable of depicting changes in white matter that are not detected with routine pulse sequences and because the findings are correlated with disability scores, the use of MR spectroscopy may prove valuable in monitoring patients after treatment and in formulating their prognosis.
Diffusion tensor imaging (DTI) can utilize diffusion-weighted imaging techniques in different orientations to establish pathology along white matter tracts in the CNS. DTI can identify demyelination and loss of axons along tracts that would otherwise go undetected by conventional techniques.[31, 32, 33] DTI can also identify disease activity in and injury to gray matter structures, which in turn can be used as a markers of disease activity and severity.[34, 35, 36, 37]
Double inversion recover (DIR) sequences can also detect cortical lesions with increased sensitivity over standard MRI sequences, with higher MRI field strengths improving sensitivity.[38]
Magnetization transfer imaging (MTI) is capable of identifying MS lesions before they can be detected by conventional MRI techniques.[39, 40]
O'Riordan et al prospectively found that in 3 of 27 patients with normal MRI findings, MS subsequently developed.[17] However, the patients with normal MRI findings all developed lesions detectable on MRI scans when the disease became established. Similarly, as patients are followed for longer periods, the rate of false-positive findings decreases, because in many patients with abnormal MRI findings after a single neurologic event, the clinical criteria for MS eventually develop.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF), also called nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MR angiography scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
Another major use of MRI has been the evaluation of patients who have had only 1 episode of neurologic impairment and who do not meet the clinical criteria for the diagnosis. The overall risk of developing MS after a single episode of neurologic impairment is estimated to be as low as 12% (2y follow-up study by Beck et al) to as high as 45% (12.9y follow-up study by Sandberg-Wollheim et al[15] ) or 58% (14.9y follow-up study by Rizzo et al[16] ).
MRI has been proven to be the most useful investigation for predicting the progression to MS. In a 10-year follow-up study of patients with a clinically isolated event, 45 (83%) of 54 patients with abnormal MRI findings went on to develop clinical MS, whereas only 3 of 27 with normal MRI findings developed MS.[17]
Degree of confidence
Tintoré et al followed up 70 patients for an average of 28.3 months after an isolated neurologic event and compared various MRI criteria for the diagnosis MS, as defined by Paty et al, Fazekas et al, and Barkhof et al.[1, 2, 18, 19] With the method of Paty et al, which requires 3 or 4 lesions (1 of which is periventricular), the authors reported a sensitivity of 86% but a specificity of only 54%.The criteria of Fazekas et al resulted in the same sensitivity and specificity. These criteria require 3 lesions with 2 of the 3 following characteristics: infratentorial location, periventricular location, and lesion greater than 6mm. The criteria of Barkhof require 1 infratentorial lesion, 1 juxtacortical lesion, 3 periventricular lesions, and either 1 gadolinium-enhanced lesion or more than 9 lesions on T2-weighted MRI scans. These criteria resulted in a sensitivity of 73% and a specificity of 73%. Thus, as the MRI criteria become more stringent in the diagnosis of MS, specificity increases at the expense of decreasing sensitivity.
In a cohort of the BENEFIT study (a multicenter, randomized, clinical study of 468 patients), the modified Barkhof criteria showed moderate predictive value for conversion to CDMS over 3 years, despite the fact that all patients received interferon beta-1b therapy for at least 1 year. Follow-up MRI was found to be most informative after 9 months in patients without dissemination in space at baseline. The overall conversion rate to CDMS was 42%. Barkhof criteria with the strongest prognostic value were the presence at baseline of at least 9 T2-weighted lesions and at least 3 periventricular lesions.[20]
According to a study of postmortem MS tissue by Pitt et al, 3-dimensional (3-D), T2*-weighted, gradient-echo (T2*GRE) and white matter–attenuated, turbo-field-echo (TFE) sequences at a 7T field strength can detect most cortical lesions. The 3-D T2*GRE and white matter–attenuated TFE sequences retrospectively detected 93% and 82% of all cortical lesions, respectively.[21]
Typical findings and pulse sequences
Because of the inflammation and breakdown of the blood-brain barrier in MS lesions, the presence of extravascular fluid leads to hyperintensity on T2-weighted images. Thus, in a patient with MS, MRI scans typically demonstrate more than 1 hyperintense white matter lesion.[22, 23, 24, 25, 26]Lesions may be observed anywhere in the CNS white matter, including the supratentorium, infratentorium, and spinal cord; however, more typical locations for MS lesions include the periventricular white matter, brainstem, cerebellum, and spinal cord. Ovoid lesions perpendicular to the ventricles are common in MS and occasionally are called Dawson bars or fingers, which occur along the path of the deep medullary veins. Perhaps the most specific lesions in MS are noted in the corpus callosum at the interface with the septum pellucidum.[27] The imaging characteristics of MS are depicted on the MRI scans below.
 Sagittal T1-weighted MRI depicts multiple hypointense lesions in the corpus callosum; this finding is characteristic of multiple sclerosis.
Sagittal T1-weighted MRI depicts multiple hypointense lesions in the corpus callosum; this finding is characteristic of multiple sclerosis.  Axial T2-weighted MRI in a patient with multiple sclerosis demonstrates numerous white matter plaques in a callosal and pericallosal white matter distribution.
Axial T2-weighted MRI in a patient with multiple sclerosis demonstrates numerous white matter plaques in a callosal and pericallosal white matter distribution.  Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis demonstrates several intensely enhancing pericallosal white matter lesions compatible with active disease.
Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis demonstrates several intensely enhancing pericallosal white matter lesions compatible with active disease.  Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity.
Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity.  Axial proton density–weighted MRI through the posterior fossa in a patient with multiple sclerosis demonstrates multiple bright foci in the brainstem and cerebellum. Proton density–weighted sequences are highly sensitive for the detection of plaques in multiple sclerosis, especially in the posterior fossa.
Axial proton density–weighted MRI through the posterior fossa in a patient with multiple sclerosis demonstrates multiple bright foci in the brainstem and cerebellum. Proton density–weighted sequences are highly sensitive for the detection of plaques in multiple sclerosis, especially in the posterior fossa.  Axial proton density–weighted MRI demonstrates multiple lesions in a distribution characteristic of multiple sclerosis. Specifically, the periventricular lesions and the more peripheral white matter lesions near the gray matter–white matter junction are typical MRI findings in multiple sclerosis.
Axial proton density–weighted MRI demonstrates multiple lesions in a distribution characteristic of multiple sclerosis. Specifically, the periventricular lesions and the more peripheral white matter lesions near the gray matter–white matter junction are typical MRI findings in multiple sclerosis.  Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis depicts enhancement of a plaque in the right temporo-occipital lobe, signifying disease activity. Note the C-shaped, or arclike, enhancement, which is fairly characteristic of multiple sclerosis.
Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis depicts enhancement of a plaque in the right temporo-occipital lobe, signifying disease activity. Note the C-shaped, or arclike, enhancement, which is fairly characteristic of multiple sclerosis.  Sagittal proton density–weighted MRI in a patient with multiple sclerosis demonstrates the characteristic corpus callosal and pericallosal white matter lesions.
Sagittal proton density–weighted MRI in a patient with multiple sclerosis demonstrates the characteristic corpus callosal and pericallosal white matter lesions.  Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis depicts several enhancing lesions, at least 2 of which show characteristic C-shaped, or arclike, peripheral enhancement.
Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis depicts several enhancing lesions, at least 2 of which show characteristic C-shaped, or arclike, peripheral enhancement.  Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity. Proton density (PD)–weighted MRI has an advantage over standard T2 imaging, because on PD series, MS lesions remain hyperintense, while the CSF signal is suppressed. Therefore, the lesions are easily identified. Depending on the PD technique, the CSF signal is suppressed to a variable degree, rendering it isointense to hypointense relative to the brain parenchyma. This sequence results in substantial suppression of Virchow-Robin spaces, which are perivascular CSF spaces that may penetrate to the subcortical white matter. These spaces may appear as hyperintense spots on standard T2-weighted MRI scans.
Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity. Proton density (PD)–weighted MRI has an advantage over standard T2 imaging, because on PD series, MS lesions remain hyperintense, while the CSF signal is suppressed. Therefore, the lesions are easily identified. Depending on the PD technique, the CSF signal is suppressed to a variable degree, rendering it isointense to hypointense relative to the brain parenchyma. This sequence results in substantial suppression of Virchow-Robin spaces, which are perivascular CSF spaces that may penetrate to the subcortical white matter. These spaces may appear as hyperintense spots on standard T2-weighted MRI scans. Compared with other techniques, nonenhanced T1-weighted MRI is far less sensitive in detecting MS lesions. Acute lesions usually are not depicted at all. With T1-weighted MRI, the clinician can gain a general appreciation of the global cerebral atrophy that occurs with advanced chronic MS. Global atrophy has been suggested to have the strongest imaging correlation with disability.
Chronic MS lesions usually result in localized leukomalacia, and they may appear as hypointense lesions that represent loss of tissue.
Gadolinium-enhanced T1-weighted MRI scans can depict acute, active MS lesions. These appear as enhancing white matter lesions; the presence of an enhancing lesion has been shown to increase the specificity for MS.[2, 18]
FLAIR MRI
Newer MRI pulse sequences and techniques, including fluid-attenuated inversion recovery (FLAIR) MRI and MR spectroscopy, have emerged that are potentially useful in the evaluation of patients with MS.FLAIR MRI is a heavily T2-weighted technique that dampens the ventricular (ie, free-water) CSF signal. Thus, the highest signals on the sequence are from certain brain parenchymal abnormalities, such as MS lesions, while the CSF appears black. This appearance is different from that on PD-weighted MRIs, on which periventricular MS lesions may appear nearly isointense to the adjacent CSF. (See the image below.)
 Coronal fluid-attenuated inversion recovery (FLAIR) MRI in a patient with multiple sclerosis demonstrates periventricular high–signal intensity lesions, which exhibit a typical distribution for multiple sclerosis. FLAIR MRI is a highly sensitive sequence for lesion detection, particularly supratentorially. The greater relative suppression of CSF on FLAIR images compared with PD-weighted series increases the contrast between periventricular lesions and CSF, enhancing their detection. FLAIR has been shown to be superior to PD-weighted sequences in the detection of MS lesions in the cerebral hemispheres. However, PD-weighted imaging remains the investigation of choice for infratentorial lesions.[28]
Coronal fluid-attenuated inversion recovery (FLAIR) MRI in a patient with multiple sclerosis demonstrates periventricular high–signal intensity lesions, which exhibit a typical distribution for multiple sclerosis. FLAIR MRI is a highly sensitive sequence for lesion detection, particularly supratentorially. The greater relative suppression of CSF on FLAIR images compared with PD-weighted series increases the contrast between periventricular lesions and CSF, enhancing their detection. FLAIR has been shown to be superior to PD-weighted sequences in the detection of MS lesions in the cerebral hemispheres. However, PD-weighted imaging remains the investigation of choice for infratentorial lesions.[28] MR spectroscopy
Magnetic resonance (MR) spectroscopy uses the characteristic spectra of specific biochemical markers to quantitate organic compounds in vivo. N -acetylaspartate (NAA) is a relatively specific neuronal marker that is present in sufficient concentrations in the brain to be revealed on MR spectroscopic images. By comparing the spectral signal of NAA with that of creatinine (Cr), MR spectroscopic can be useful in assessing neuronal and axonal loss.Arnold et al noted that the NAA-Cr ratio in the CNS was decreased in moderate to advanced MS. White matter that appeared normal on T1- and T2-weighted images also demonstrated the reduction.[29] In addition, a normal ratio was noted in the area of a recently active lesion associated with clinical deficits that subsequently resolved. The findings led the authors to propose that MR spectroscopic findings may be able to help identify irreversible axonal damage.
In a study involving 88 patients with MS, De Stefano et al found a strong correlation between disability scores and NAA-Cr ratios.[30] The ratio exhibited a stronger correlation in patients with MS patients who had milder disability scores. Because MR spectroscopy appears to be capable of depicting changes in white matter that are not detected with routine pulse sequences and because the findings are correlated with disability scores, the use of MR spectroscopy may prove valuable in monitoring patients after treatment and in formulating their prognosis.
Nonstandard MRI sequences
Beyond the standard MRI sequences that are used in clinical practice (T1 +/- Gad, T2, diffusion-weighted imaging, FLAIR), more advanced MRI techniques have been used for research purposes for several years. Many of these series require greater magnetic field strengths over the popular 1.5T, but with the increasing availability of 3T MRI, these sequences will likely find their way more and more into standard clinical practice.Diffusion tensor imaging (DTI) can utilize diffusion-weighted imaging techniques in different orientations to establish pathology along white matter tracts in the CNS. DTI can identify demyelination and loss of axons along tracts that would otherwise go undetected by conventional techniques.[31, 32, 33] DTI can also identify disease activity in and injury to gray matter structures, which in turn can be used as a markers of disease activity and severity.[34, 35, 36, 37]
Double inversion recover (DIR) sequences can also detect cortical lesions with increased sensitivity over standard MRI sequences, with higher MRI field strengths improving sensitivity.[38]
Magnetization transfer imaging (MTI) is capable of identifying MS lesions before they can be detected by conventional MRI techniques.[39, 40]
Limitations
In virtually all patients with clinically well-established MS, MRI scans demonstrate the corresponding changes. False-negative findings occur more frequently in patients with early MS and a minimal clinical history of neurologic impairment than in other patients.O'Riordan et al prospectively found that in 3 of 27 patients with normal MRI findings, MS subsequently developed.[17] However, the patients with normal MRI findings all developed lesions detectable on MRI scans when the disease became established. Similarly, as patients are followed for longer periods, the rate of false-positive findings decreases, because in many patients with abnormal MRI findings after a single neurologic event, the clinical criteria for MS eventually develop.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF), also called nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MR angiography scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
Brain Imaging in Multiple Sclerosis _Ultrasonography
To contact us Click HERE
Ultrasonography is not currently used in the investigation of MS. Berg et al, however, used transcranial sonography to determine the size of the ventricles in patients with MS and found that an increasing size is correlated with the MRI-determined brain volume, as well as with cognitive dysfunction and clinical disability. Further studies may establish a role for ultrasonography in the prognosis and treatment of patients with MS.[41]
According to Walter et al, in patients with MS, hyperechogenicity of the substantia nigra and lenticular nucleus correlates with more pronounced MRI T2 hypointensity, which is thought to reflect iron deposition, and a larger bilateral substantia nigra echogenic area is related to a higher rate of disease progression. In addition, a small echogenic area predicts a disease course without further progression over 2 years.[42]
Walter et al performed the study to determine whether transcranial ultrasonography can identify lesions in deep gray matter in patients with MS and whether such findings can identify the severity and progression of MS. Of 75 patients followed, abnormal hyperechogenicity of the substantia nigra occurred in 41%; of the lenticular nucleus, in 54%; of the caudate nucleus, in 40%; and of the thalamus, in 8%, with similar frequency in patients with relapsing-remitting and primary or secondary progressive MS if corrected for disease duration.
According to Walter et al, in patients with MS, hyperechogenicity of the substantia nigra and lenticular nucleus correlates with more pronounced MRI T2 hypointensity, which is thought to reflect iron deposition, and a larger bilateral substantia nigra echogenic area is related to a higher rate of disease progression. In addition, a small echogenic area predicts a disease course without further progression over 2 years.[42]
Walter et al performed the study to determine whether transcranial ultrasonography can identify lesions in deep gray matter in patients with MS and whether such findings can identify the severity and progression of MS. Of 75 patients followed, abnormal hyperechogenicity of the substantia nigra occurred in 41%; of the lenticular nucleus, in 54%; of the caudate nucleus, in 40%; and of the thalamus, in 8%, with similar frequency in patients with relapsing-remitting and primary or secondary progressive MS if corrected for disease duration.
Pituitary Adenoma Imaging
To contact us Click HERE
For pituitary adenoma imaging, CT and MRI have largely replaced plain radiography because conventional radiography is poor for delineating soft tissues (see Preferred Examination, below), as well as CT and MRI sections).[1, 2, 3, 4, 5, 6, 7]
The pituitary gland is the master gland of the body because it controls most of the body's endocrine functions by means of the hypothalamic-pituitary axis (see the images below). The anterior lobe of the pituitary gland secretes 6 hormones: thyroid-stimulating hormone (TSH), previously adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), leuteinizing hormone (LH), growth hormone (GH), and prolactin (PRL). The posterior pituitary gland secretes vasopressin and oxytocin.
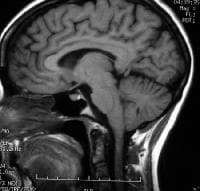 T1-weighted sagittal MRI through the pituitary fossa shows a normal, isointense anterior pituitary and a hyperintense posterior pituitary gland.
T1-weighted sagittal MRI through the pituitary fossa shows a normal, isointense anterior pituitary and a hyperintense posterior pituitary gland.  Lateral skull radiograph in a patient with pituitary adenoma shows an enlarged sella and focal calcification in the adenoma (arrow) Pituitary adenomas are almost always benign with no malignant potential. In general, pituitary lesions can be subdivided into nonsecretory and secretory tumors of the pituitary gland, other intrasellar tumors, and parasellar tumors. The last group occurs in the vicinity of the sellar turcica and can mimic the pituitary tumors in terms of the symptoms they cause. Nonsecretory pituitary tumors are called null-cell tumors. Small null-cell tumors measuring a few millimeters are common and found in up to 25% of autopsied pituitary glands. These may grow slowly, destroying normal pituitary function (hypopituitarism), or they may compress nearby structures and cause neurologic problems.
Lateral skull radiograph in a patient with pituitary adenoma shows an enlarged sella and focal calcification in the adenoma (arrow) Pituitary adenomas are almost always benign with no malignant potential. In general, pituitary lesions can be subdivided into nonsecretory and secretory tumors of the pituitary gland, other intrasellar tumors, and parasellar tumors. The last group occurs in the vicinity of the sellar turcica and can mimic the pituitary tumors in terms of the symptoms they cause. Nonsecretory pituitary tumors are called null-cell tumors. Small null-cell tumors measuring a few millimeters are common and found in up to 25% of autopsied pituitary glands. These may grow slowly, destroying normal pituitary function (hypopituitarism), or they may compress nearby structures and cause neurologic problems.
Functioning pituitary adenomas can be clinically classified by means of the hormone they elaborate. These tumors become symptomatic because they secrete hormones, and they are less likely than like null-cell tumors to become large enough to compress adjacent structures. As pituitary tumors grow, destruction of normal pituitary tissue results in various hormonal deficiencies. In rare cases, these tumors may spontaneously hemorrhage or become infarcted. The pressure they exert on nearby structures can produce double vision and facial numbness. The optic chiasm is directly above the pituitary gland, and upward growth of pituitary tumors frequently causes progressive visual loss. This visual loss typically begins from each side of the field of vision and leads to tunnel vision and then blindness.[8, 9]
The clinical features of pituitary adenoma vary depending on the location and size of the tumor and its secretory capability. Pituitary adenomas typically appear during early adulthood, and no sex predilection is known. Secretory pituitary adenomas are usually small and generally do not cause neurologic symptoms or hypopituitarism, though they can. The symptoms of functioning tumors are related to the specific hormone the tumor produces.[10, 11]
Neurologic symptoms of pituitary adenomas include headaches; double vision; and loss of peripheral vision leading to blindness, facial pain, or numbness. Hypopituitarism manifests itself by lack of energy, weight loss, nausea, vomiting, constipation, amenorrhea and infertility, dry skin, increase pigmentation of the skin, cold intolerance, and mental status changes (eg, sleepiness, psychosis, collapse).
A prolactinoma is the most common pituitary tumor and may cause amenorrhea, irregular periods, galactorrhea, infertility in women and osteoporosis. It may cause hypogonadism, loss of libido, and impotence in men.
Tumors that secrete excess GH cause gigantism in children and acromegaly in adults. Acromegaly is associated with enlargement of facial features, hands and feet, heart disease, hypertension, arthritis, carpel tunnel syndrome, amenorrhea, and impotence.
ACTH-secreting adenomas produce Cushing disease, which itself results in a widened face with acne and flushing, fatty deposits over the back of the neck, stretch marks, easy bruising, hair growth, diabetes mellitus, muscle loss, fatigue, depression, and psychosis.
Tumors that elaborate TSH produce signs and symptoms of thyrotoxicosis, such as heat intolerance, sweating, tachycardia, fine tremor, and weight loss. Some tumors may secrete more than one hormone, such as GH and PRL.
Rare tumors secrete LH or FSH (gonadotrophins). When pituitary tumors compromise the secretory cells, the first evidence of cellular failure usually affects the gonadotrophins. Therefore, the disappearance of menstrual periods may be the first sign of a pituitary tumor in female patients. In male patients, the most common symptom of deficiency is impotence. Isolated deficiencies of both LH and FSH do occur, but only rarely. In a male individual, LH deficiency alone leads to the appearance of a fertile eunuch. In this condition, sufficient FSH is present to permit the maturation of spermatozoa; however, because of the LH deficiency, the patient has many of the characteristics of a castrated individual. Tumors also can produce an excess of LH or FSH, and pituitary tumors that secrete only the nonspecific, hormonally inactive alpha unit of glycoprotein hormones are not rare.
Other features
Visual symptoms are generally related to compression of visual pathways and include bitemporal visual-field loss, which is denser from the superior to inferior than in other orientations, color desaturation, diplopia, and ophthalmoplegia. The funduscopic sign of long-standing chiasmal compression is primary optic atrophy. Severe optic atrophy indicates a poor prognosis for visual recovery after surgical decompression. In pregnant women, bitemporal visual-field loss and headache may indicate pituitary apoplexy.
Pituitary apoplexy is a potentially life-threatening condition. Women with pituitary adenomas and MRI evidence of subarachnoid bleeding should deliver by cesarean section to prevent apoplexy during delivery. Postpartum hemorrhage can cause infarction of the pituitary gland, leading to hypopituitarism (Sheehan syndrome).
Clinical masquerades of pituitary adenoma include chronic retrobulbar optic neuritis, nutritional amblyopia, uncorrected refractive error, normal-tension glaucoma, and age-related maculopathy. Bilateral tilted-disc syndrome can result in a superior bitemporal field defect similar to that observed in pituitary adenoma. However, the field defect in tilted-disc syndrome is unchanging and does not respect the vertical midline, whereas the field defects in chiasm-compressive lesions are progressive and do respect the vertical midline.
Craniopharyngioma
Craniopharyngiomas are benign, slow-growing tumors that originate from epithelial remnants of the Rathke pouch at the junction of the infundibulum and the pituitary gland. These lesions are composed of both solid epithelial tissue and cystic components. The cystic components contain variable amounts of cholesterol, keratin, necrotic debris, proteinaceous fluid, and hemorrhage. Calcification is present in 75-85%.
MRI appearances vary depending on the amount of solid and cystic components and on the nature of cystic contents. Heterogeneous signal intensity on images obtained with all sequences is the most typical finding. Solid components are hypointense on T1-weighted images and hyperintense on T2-weighted images. The cysts also have a long T2; however, if they have a high cholesterol content or methemoglobin, shortening of T1 results in high signal intensity on T1-weighted images. Calcification in the tumor is better detected with CT than with MRI because MRI may not reliably depict calcification. Craniopharyngioma may also cause truncation of the dorsum sellae and upward growth into the third ventricle, which is readily identified on MRI.
Aneurysm
Aneurysms in the parasellar region may originate from the circle of Willis or intracavernous carotid arteries. MRI features include a mass of heterogeneous signal intensity due to flow effects and thrombus formation. Low signal intensity is caused by high flow and chronic thrombus; high signal intensity may represent slow flow or subacute thrombus. Flow in the patent lumen may also cause a band of artifact in the phase-encoding direction on spin-echo images. Magnetic resonance angiography (MRA) is useful in confirming an aneurysm. A potential pitfall in diagnosis is a pneumatized anterior clinoid or calcification, which can simulate the flow void of an aneurysm.
Empty Sella
An empty sella occurs as a result of herniation of the arachnoid through an incompetent diaphragma sellae. Over time, cerebrospinal fluid (CSF) pulsations may enlarge the sella and compress the gland against the floor of the sella. An empty sella is usually asymptomatic and an incidental finding, but can be a manifestation of increased intracranial pressure. However, they are occasionally severe. Compression of the pituitary gland may affect function, or traction on the optic chiasm and nerves may cause visual symptoms.
Chiasmatic and hypothalamic gliomas
Gliomas of the optic chiasm and the hypothalamic pathways are primarily tumors of children and young adults. The tumors tend to be low grade, but they infiltrate along the visual pathways. Neurofibromatosis is strongly associated with optic and chiasmatic gliomas. Hypothalamic gliomas are generally aggressive and produce symptoms early, resulting in 1 of many hypothalamic syndromes: diabetes insipidus; inappropriate secretion of antidiuretic hormone (ADH); Fröhlich syndrome; or disturbances of temperature, appetite, or metabolism.
Chiasmatic gliomas are usually isointense or slightly hypointense on T1-weighted images and hyperintense on T2-weighted images. MRI is useful in determining degree of infiltration of the optic chiasm or optic nerves and for assessing posterior extension into the lateral geniculate body and the occasional exophytic growth into the suprasellar and interpeduncular cisterns. Both hypothalamic and chiasmatic gliomas are enhancing after the intravenous administration of gadolinium-based contrast agent. The multiplanar capability of MRI enables it to depict extension into surrounding structures well.
Pituicytomas
Pituicytomas are also called choristomas, granular cell tumors, or myoblastomas and are rare largely noninfiltrating sellar or parasellar tumors in adults. They arise along the distribution of the neurohypophysis, including both the stalk and the posterior lobe. They occur in the suprasellar space, in the sella, or both. Most symptomatic pituicytomas appear as suprasellar masses. They may originate from the posterior pituitary and remain confined within the sella turcica. Pituicytoma is generally a surprise finding in that it is seldom considered in the preoperative differential diagnosis of a suprasellar lesion. The MRI signal-intensity characteristics vary depending on the cystic components. The solid parts of the tumor are enhancing.
Ectopic pituitary
An ectopic pituitary gland results in high signal intensity adjacent to the median eminence of the hypothalamus with an absence of the normal posterior pituitary bright spot on T1-weighted MRIs. An ectopic pituitary may be associated with perinatal asphyxia and disruption of the normal hypothalamic-pituitary axis. Traumatic transection of the stalk is exceptionally rare, but it can result in abnormal accumulation of posterior-lobe hormones proximal to the disruption.
Metastasis
Most metastases to the pituitary gland are small, clinically silent, and rare in the clinical setting, though they are frequently reported in autopsy series. Large metastasis may cause diabetes insipidus. Leukemia, lymphoma, and cancers of the lung or breast are the most common primary origins. The demonstration of rapid growth distinguishes metastases from slow-growing pituitary adenomas.
Rathke cleft cyst
Rathke cleft cysts arise from remnants of the Rathke cleft, a fetal link between the hypothalamus and nasopharynx that obliterates in normal individuals. Rathke cleft cysts are benign cysts lined by a single layer of ciliated columnar or cuboidal epithelium, and they often contain goblet cells. When small, Rathke cleft cysts are intrasellar. However, as they grow, they extend into the suprasellar region. Rathke cleft cysts are smoothly marginated and well-defined lesions. Their MRI signal-intensity characteristics vary depending on the contents of the cyst. After gadolinium enhancement, only the capsule is enhancing; the nodular component is not enhancing. This feature helps distinguish these lesions from craniopharyngiomas.
Hamartoma of the tuber cinereum
Hamartomas of the tuber cinereum of the hypothalamus are benign, slow-growing tumors that consist of hyperplastic hypothalamic glial and neural tissue. The usual presentation is precocious puberty. The tumors may be sessile or pedunculated, occurring between the infundibulum and mamillary bodies. They are usually isointense to the brain on T1-weighted images and mildly hyperintense on T2-weighted images. They do not show contrast enhancement, a feature that distinguishes them from hypothalamic gliomas and germinomas.
Lymphoma
Favored sites for primary malignant non-Hodgkin lymphomas are the hypothalamus, the cavernous sinuses, and the perisellar regions. These rapidly growing tumors mostly affect patients who are immunocompromised because of chemotherapy, HIV infection, or organ transplantation. Lymphomas typically appear as homogeneous, slightly hyperintense masses on T2-weighted images. In general, lymphomas are uniformly and intensely enhancing. Cystic, hemorrhagic, and necrotic areas in these tumors are unusual.
Germinoma
Germinomas occur in the pineal and suprasellar region and affect children and young adults. Because of their propensity to invade the hypothalamus and to grow into the third ventricle, they may cause endocrine dysfunction. They are known to disseminate through CSF pathways. The tumors are usually well defined and isointense with the brain on T1- and T2-weighted images. They generally do not show necrosis, cystic change, or hemorrhage. Contrast enhancement is moderate and essential in the assessment of CSF spread of the tumor. Calcification in germinomas may make them difficult to visualize on MRIs.
Arachnoid cyst
Arachnoid cysts are CSF-containing spaces that are generally not confused with pituitary or parasellar tumors. These cysts have CSF signal intensity, they are well defined, they do not calcify, and they are not enhancing after the administration of contrast material.
Epidermoid cyst
Epidermoid cysts are benign, slow-growing tumors that arise from epithelial cell rests in the basal cisterns. They have a propensity to grow along the subarachnoid spaces and into the various crevices at the base of the brain. Intradural epidermoids are usually large, with lobulated outer margins and an insinuating pattern of growth. Epidermoid cysts are classically confused with arachnoid cysts on CT and MRI because they are similar to CSF in attenuation and intensity, respectively, and because they have similar T1 and T2 signal intensities. Diffusion-weighted images, proton density–weighted images, and fluid-attenuated inversion recovery (FLAIR) images are useful in making the distinction. Epidermoid cysts do not show contrast enhancement.
Dermoid cyst
Dermoid cysts occur in the pineal and suprasellar regions, as well as other midline locations. On histology, they have both mesodermal and ectodermal derivatives, which account for their varied appearance on MRI. The MRI appearances are those of a heterogeneous tumor. Fatty tissues in the tumor produce high signal intensity on T1-weighted images, and a fat-fluid level may be seen. These cysts may rupture, causing the cystic contents to leak into a ventricle or subarachnoid space and then produce an ependymitis or meningitis, respectively.
Meningioma
Parasellar meningiomas commonly involve the cavernous sinus and produce ophthalmoplegia. Meningiomas are hypervascular tumors that derive their blood supply from the dural vessels. They also induce an osteoblastic reaction in the adjacent bone, resulting in a characteristic focal hyperostosis, which is well depicted on plain radiographs and CT. MRI signal is isointense relative to the brain on T1- and T2-weighted images. MRI is particularly good for assessing encasement of the cavernous sinus and carotid artery. The tumor is intensely enhancing on contrast-enhanced images.
Schwannomas
Schwannomas are nerve-sheath tumors that may involve cranial nerves III-XII or peripheral nerves. Schwannomas in the parasellar region arise from the trigeminal nerve and, in rare cases, from the third, fourth, or sixth cranial nerves. These tumors are benign, well encapsulated, and globular; all of these features that distinguish them from broad-based meningiomas. The tumors are isointense on T1-weighted MRIs and mildly hyperintense on T2-weighted MRIs. Cystic degeneration is frequent; hemorrhage and calcification are rare. The solid portions of these tumors are strongly enhancing with the use of intravenous contrast material.
Primary and secondary tumors of the skull base
A wide variety of primary and metastatic tumors of the skull base can involve the parasellar region. Nasopharyngeal carcinomas and malignant tumors of the paranasal sinuses may invade the parasellar region, and metastases from distant primary tumors may also involve the sphenoid bone and the parasellar region. The primary tumors include chordoma, chondroma, chondrosarcoma, and plasmacytoma.
Chordomas have uniform high signal intensity on T2-weighted MRIs. Chondrosarcomas are heterogeneous on images obtained with all sequences because of their variable calcification and chondral elements, but they usually have areas of high T2 signal intensity. Chordomas characteristically arise in the midline, whereas chondrosarcomas arise off midline at the synchondroses. Alteration of the normal hyperintense clival fat is a sensitive indicator of these tumors; therefore, nonenhanced non–fat-saturated T1-weighted imaging is particularly useful. Contrast enhancement is helpful in assessing the intracranial components of these tumors.
Carotid-cavernous fistula
The fistulous communication between the carotid artery and cavernous sinus may result from a dural arteriovenous malformation, trauma, or transsphenoidal surgery. Arterial pressures cause expansion of the cavernous sinus and dilation of parasellar and orbital veins. Proptosis, chemosis, and visual loss may complicate such fistulas. Flow effects and flow artifacts on MRI may confirm the diagnosis.
Granulomatous hypophysitis
Granulomatous hypophysitis is a rare entity that mimics a pituitary adenoma both clinically and radiologically. The reported causes of granulomatous hypophysitis include tuberculosis, syphilis, sarcoidosis, mycotic granuloma, and foreign body granuloma due to a ruptured Rathke cleft cyst. Lymphocytic hypophysitis represent inflammation of the pituitary gland and may complicate pregnancy or the postpartum period. MRI findings include diffuse enlargement of the anterior lobe and pituitary stalk. The gland is strongly enhanced with gadolinium-based contrast agents, and the enhancement commonly extends along the diaphragma sellae.
McCune-Albright syndrome
McCune-Albright syndrome (MAS) may clinically mimic acromegaly or gigantism. The syndrome is characterized by polyostotic fibrous dysplasia, café au lait pigmentation of the skin, and autonomous endocrine hyperfunction. The most common form of autonomous endocrine hyperfunction in this syndrome is gonadotropin-independent precocious puberty; however, affected individuals also may have hyperthyroidism, hypercortisolism, pituitary gigantism, or acromegaly. Nonendocrine abnormalities in this disorder include hypophosphatemia, chronic liver disease, tachycardia, and sudden death (which is rare and possibly due to cardiac arrhythmias).
Angiography is seldom performed; if indicated, CT angiography (CTA) and MRA have largely replaced conventional angiography. Angiography has a role when intervention is indicated in the cavernous sinus or in the cavernous part of the carotid artery.
Conventional single-section CT has a limited role in pituitary imaging, with a sensitivity of 17-22% in detecting microadenomas. Multidetector-row CT with 64 channels may have a role, especially in patients unable to undergo MRI. CT is best for visualizing bony detail and calcification in tumors such as germinomas, craniopharyngiomas, and meningiomas. CTA is excellent for showing the morphology of parasellar aneurysms and for presurgical planning. CT scans are valuable when MRI is contraindicated, as in patients with pacemakers or metallic implants in the brain or eyes.
MRI is generally preferred over CT for the diagnosis of pituitary adenomas because of its superior definition of small lesions in the pituitary sella and its improved anatomic definition before surgery. MRI is also preferred for postsurgical surveillance.
Somatostatin-receptor scintigraphy may be used to distinguish recurrent or residual tumor from scar or necrotic tissue after surgery.
A potential pitfall of MRI diagnosis is a pneumatized anterior clinoid or calcification, which can simulate the flow void of an aneurysm. In addition, MRI is contraindicated in patients with pacemakers or ferromagnetic implants in the brain or eyes. With CT or MRI, pituitary adenoma remnants can be hard to differentiate from radiotherapy-induced fibrosis, especially in patients with clinically nonfunctioning pituitary adenomas, who lack circulating markers that aid in monitoring the progression or cure of the disease.
The pituitary gland is the master gland of the body because it controls most of the body's endocrine functions by means of the hypothalamic-pituitary axis (see the images below). The anterior lobe of the pituitary gland secretes 6 hormones: thyroid-stimulating hormone (TSH), previously adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), leuteinizing hormone (LH), growth hormone (GH), and prolactin (PRL). The posterior pituitary gland secretes vasopressin and oxytocin.
 T1-weighted sagittal MRI through the pituitary fossa shows a normal, isointense anterior pituitary and a hyperintense posterior pituitary gland.
T1-weighted sagittal MRI through the pituitary fossa shows a normal, isointense anterior pituitary and a hyperintense posterior pituitary gland.  Lateral skull radiograph in a patient with pituitary adenoma shows an enlarged sella and focal calcification in the adenoma (arrow) Pituitary adenomas are almost always benign with no malignant potential. In general, pituitary lesions can be subdivided into nonsecretory and secretory tumors of the pituitary gland, other intrasellar tumors, and parasellar tumors. The last group occurs in the vicinity of the sellar turcica and can mimic the pituitary tumors in terms of the symptoms they cause. Nonsecretory pituitary tumors are called null-cell tumors. Small null-cell tumors measuring a few millimeters are common and found in up to 25% of autopsied pituitary glands. These may grow slowly, destroying normal pituitary function (hypopituitarism), or they may compress nearby structures and cause neurologic problems.
Lateral skull radiograph in a patient with pituitary adenoma shows an enlarged sella and focal calcification in the adenoma (arrow) Pituitary adenomas are almost always benign with no malignant potential. In general, pituitary lesions can be subdivided into nonsecretory and secretory tumors of the pituitary gland, other intrasellar tumors, and parasellar tumors. The last group occurs in the vicinity of the sellar turcica and can mimic the pituitary tumors in terms of the symptoms they cause. Nonsecretory pituitary tumors are called null-cell tumors. Small null-cell tumors measuring a few millimeters are common and found in up to 25% of autopsied pituitary glands. These may grow slowly, destroying normal pituitary function (hypopituitarism), or they may compress nearby structures and cause neurologic problems. Functioning pituitary adenomas can be clinically classified by means of the hormone they elaborate. These tumors become symptomatic because they secrete hormones, and they are less likely than like null-cell tumors to become large enough to compress adjacent structures. As pituitary tumors grow, destruction of normal pituitary tissue results in various hormonal deficiencies. In rare cases, these tumors may spontaneously hemorrhage or become infarcted. The pressure they exert on nearby structures can produce double vision and facial numbness. The optic chiasm is directly above the pituitary gland, and upward growth of pituitary tumors frequently causes progressive visual loss. This visual loss typically begins from each side of the field of vision and leads to tunnel vision and then blindness.[8, 9]
Clinical features
Features based on secretory abilityThe clinical features of pituitary adenoma vary depending on the location and size of the tumor and its secretory capability. Pituitary adenomas typically appear during early adulthood, and no sex predilection is known. Secretory pituitary adenomas are usually small and generally do not cause neurologic symptoms or hypopituitarism, though they can. The symptoms of functioning tumors are related to the specific hormone the tumor produces.[10, 11]
Neurologic symptoms of pituitary adenomas include headaches; double vision; and loss of peripheral vision leading to blindness, facial pain, or numbness. Hypopituitarism manifests itself by lack of energy, weight loss, nausea, vomiting, constipation, amenorrhea and infertility, dry skin, increase pigmentation of the skin, cold intolerance, and mental status changes (eg, sleepiness, psychosis, collapse).
A prolactinoma is the most common pituitary tumor and may cause amenorrhea, irregular periods, galactorrhea, infertility in women and osteoporosis. It may cause hypogonadism, loss of libido, and impotence in men.
Tumors that secrete excess GH cause gigantism in children and acromegaly in adults. Acromegaly is associated with enlargement of facial features, hands and feet, heart disease, hypertension, arthritis, carpel tunnel syndrome, amenorrhea, and impotence.
ACTH-secreting adenomas produce Cushing disease, which itself results in a widened face with acne and flushing, fatty deposits over the back of the neck, stretch marks, easy bruising, hair growth, diabetes mellitus, muscle loss, fatigue, depression, and psychosis.
Tumors that elaborate TSH produce signs and symptoms of thyrotoxicosis, such as heat intolerance, sweating, tachycardia, fine tremor, and weight loss. Some tumors may secrete more than one hormone, such as GH and PRL.
Rare tumors secrete LH or FSH (gonadotrophins). When pituitary tumors compromise the secretory cells, the first evidence of cellular failure usually affects the gonadotrophins. Therefore, the disappearance of menstrual periods may be the first sign of a pituitary tumor in female patients. In male patients, the most common symptom of deficiency is impotence. Isolated deficiencies of both LH and FSH do occur, but only rarely. In a male individual, LH deficiency alone leads to the appearance of a fertile eunuch. In this condition, sufficient FSH is present to permit the maturation of spermatozoa; however, because of the LH deficiency, the patient has many of the characteristics of a castrated individual. Tumors also can produce an excess of LH or FSH, and pituitary tumors that secrete only the nonspecific, hormonally inactive alpha unit of glycoprotein hormones are not rare.
Other features
Visual symptoms are generally related to compression of visual pathways and include bitemporal visual-field loss, which is denser from the superior to inferior than in other orientations, color desaturation, diplopia, and ophthalmoplegia. The funduscopic sign of long-standing chiasmal compression is primary optic atrophy. Severe optic atrophy indicates a poor prognosis for visual recovery after surgical decompression. In pregnant women, bitemporal visual-field loss and headache may indicate pituitary apoplexy.
Pituitary apoplexy is a potentially life-threatening condition. Women with pituitary adenomas and MRI evidence of subarachnoid bleeding should deliver by cesarean section to prevent apoplexy during delivery. Postpartum hemorrhage can cause infarction of the pituitary gland, leading to hypopituitarism (Sheehan syndrome).
Other problems to consider
Clinical masqueradesClinical masquerades of pituitary adenoma include chronic retrobulbar optic neuritis, nutritional amblyopia, uncorrected refractive error, normal-tension glaucoma, and age-related maculopathy. Bilateral tilted-disc syndrome can result in a superior bitemporal field defect similar to that observed in pituitary adenoma. However, the field defect in tilted-disc syndrome is unchanging and does not respect the vertical midline, whereas the field defects in chiasm-compressive lesions are progressive and do respect the vertical midline.
Craniopharyngioma
Craniopharyngiomas are benign, slow-growing tumors that originate from epithelial remnants of the Rathke pouch at the junction of the infundibulum and the pituitary gland. These lesions are composed of both solid epithelial tissue and cystic components. The cystic components contain variable amounts of cholesterol, keratin, necrotic debris, proteinaceous fluid, and hemorrhage. Calcification is present in 75-85%.
MRI appearances vary depending on the amount of solid and cystic components and on the nature of cystic contents. Heterogeneous signal intensity on images obtained with all sequences is the most typical finding. Solid components are hypointense on T1-weighted images and hyperintense on T2-weighted images. The cysts also have a long T2; however, if they have a high cholesterol content or methemoglobin, shortening of T1 results in high signal intensity on T1-weighted images. Calcification in the tumor is better detected with CT than with MRI because MRI may not reliably depict calcification. Craniopharyngioma may also cause truncation of the dorsum sellae and upward growth into the third ventricle, which is readily identified on MRI.
Aneurysm
Aneurysms in the parasellar region may originate from the circle of Willis or intracavernous carotid arteries. MRI features include a mass of heterogeneous signal intensity due to flow effects and thrombus formation. Low signal intensity is caused by high flow and chronic thrombus; high signal intensity may represent slow flow or subacute thrombus. Flow in the patent lumen may also cause a band of artifact in the phase-encoding direction on spin-echo images. Magnetic resonance angiography (MRA) is useful in confirming an aneurysm. A potential pitfall in diagnosis is a pneumatized anterior clinoid or calcification, which can simulate the flow void of an aneurysm.
Empty Sella
An empty sella occurs as a result of herniation of the arachnoid through an incompetent diaphragma sellae. Over time, cerebrospinal fluid (CSF) pulsations may enlarge the sella and compress the gland against the floor of the sella. An empty sella is usually asymptomatic and an incidental finding, but can be a manifestation of increased intracranial pressure. However, they are occasionally severe. Compression of the pituitary gland may affect function, or traction on the optic chiasm and nerves may cause visual symptoms.
Chiasmatic and hypothalamic gliomas
Gliomas of the optic chiasm and the hypothalamic pathways are primarily tumors of children and young adults. The tumors tend to be low grade, but they infiltrate along the visual pathways. Neurofibromatosis is strongly associated with optic and chiasmatic gliomas. Hypothalamic gliomas are generally aggressive and produce symptoms early, resulting in 1 of many hypothalamic syndromes: diabetes insipidus; inappropriate secretion of antidiuretic hormone (ADH); Fröhlich syndrome; or disturbances of temperature, appetite, or metabolism.
Chiasmatic gliomas are usually isointense or slightly hypointense on T1-weighted images and hyperintense on T2-weighted images. MRI is useful in determining degree of infiltration of the optic chiasm or optic nerves and for assessing posterior extension into the lateral geniculate body and the occasional exophytic growth into the suprasellar and interpeduncular cisterns. Both hypothalamic and chiasmatic gliomas are enhancing after the intravenous administration of gadolinium-based contrast agent. The multiplanar capability of MRI enables it to depict extension into surrounding structures well.
Pituicytomas
Pituicytomas are also called choristomas, granular cell tumors, or myoblastomas and are rare largely noninfiltrating sellar or parasellar tumors in adults. They arise along the distribution of the neurohypophysis, including both the stalk and the posterior lobe. They occur in the suprasellar space, in the sella, or both. Most symptomatic pituicytomas appear as suprasellar masses. They may originate from the posterior pituitary and remain confined within the sella turcica. Pituicytoma is generally a surprise finding in that it is seldom considered in the preoperative differential diagnosis of a suprasellar lesion. The MRI signal-intensity characteristics vary depending on the cystic components. The solid parts of the tumor are enhancing.
Ectopic pituitary
An ectopic pituitary gland results in high signal intensity adjacent to the median eminence of the hypothalamus with an absence of the normal posterior pituitary bright spot on T1-weighted MRIs. An ectopic pituitary may be associated with perinatal asphyxia and disruption of the normal hypothalamic-pituitary axis. Traumatic transection of the stalk is exceptionally rare, but it can result in abnormal accumulation of posterior-lobe hormones proximal to the disruption.
Metastasis
Most metastases to the pituitary gland are small, clinically silent, and rare in the clinical setting, though they are frequently reported in autopsy series. Large metastasis may cause diabetes insipidus. Leukemia, lymphoma, and cancers of the lung or breast are the most common primary origins. The demonstration of rapid growth distinguishes metastases from slow-growing pituitary adenomas.
Rathke cleft cyst
Rathke cleft cysts arise from remnants of the Rathke cleft, a fetal link between the hypothalamus and nasopharynx that obliterates in normal individuals. Rathke cleft cysts are benign cysts lined by a single layer of ciliated columnar or cuboidal epithelium, and they often contain goblet cells. When small, Rathke cleft cysts are intrasellar. However, as they grow, they extend into the suprasellar region. Rathke cleft cysts are smoothly marginated and well-defined lesions. Their MRI signal-intensity characteristics vary depending on the contents of the cyst. After gadolinium enhancement, only the capsule is enhancing; the nodular component is not enhancing. This feature helps distinguish these lesions from craniopharyngiomas.
Hamartoma of the tuber cinereum
Hamartomas of the tuber cinereum of the hypothalamus are benign, slow-growing tumors that consist of hyperplastic hypothalamic glial and neural tissue. The usual presentation is precocious puberty. The tumors may be sessile or pedunculated, occurring between the infundibulum and mamillary bodies. They are usually isointense to the brain on T1-weighted images and mildly hyperintense on T2-weighted images. They do not show contrast enhancement, a feature that distinguishes them from hypothalamic gliomas and germinomas.
Lymphoma
Favored sites for primary malignant non-Hodgkin lymphomas are the hypothalamus, the cavernous sinuses, and the perisellar regions. These rapidly growing tumors mostly affect patients who are immunocompromised because of chemotherapy, HIV infection, or organ transplantation. Lymphomas typically appear as homogeneous, slightly hyperintense masses on T2-weighted images. In general, lymphomas are uniformly and intensely enhancing. Cystic, hemorrhagic, and necrotic areas in these tumors are unusual.
Germinoma
Germinomas occur in the pineal and suprasellar region and affect children and young adults. Because of their propensity to invade the hypothalamus and to grow into the third ventricle, they may cause endocrine dysfunction. They are known to disseminate through CSF pathways. The tumors are usually well defined and isointense with the brain on T1- and T2-weighted images. They generally do not show necrosis, cystic change, or hemorrhage. Contrast enhancement is moderate and essential in the assessment of CSF spread of the tumor. Calcification in germinomas may make them difficult to visualize on MRIs.
Arachnoid cyst
Arachnoid cysts are CSF-containing spaces that are generally not confused with pituitary or parasellar tumors. These cysts have CSF signal intensity, they are well defined, they do not calcify, and they are not enhancing after the administration of contrast material.
Epidermoid cyst
Epidermoid cysts are benign, slow-growing tumors that arise from epithelial cell rests in the basal cisterns. They have a propensity to grow along the subarachnoid spaces and into the various crevices at the base of the brain. Intradural epidermoids are usually large, with lobulated outer margins and an insinuating pattern of growth. Epidermoid cysts are classically confused with arachnoid cysts on CT and MRI because they are similar to CSF in attenuation and intensity, respectively, and because they have similar T1 and T2 signal intensities. Diffusion-weighted images, proton density–weighted images, and fluid-attenuated inversion recovery (FLAIR) images are useful in making the distinction. Epidermoid cysts do not show contrast enhancement.
Dermoid cyst
Dermoid cysts occur in the pineal and suprasellar regions, as well as other midline locations. On histology, they have both mesodermal and ectodermal derivatives, which account for their varied appearance on MRI. The MRI appearances are those of a heterogeneous tumor. Fatty tissues in the tumor produce high signal intensity on T1-weighted images, and a fat-fluid level may be seen. These cysts may rupture, causing the cystic contents to leak into a ventricle or subarachnoid space and then produce an ependymitis or meningitis, respectively.
Meningioma
Parasellar meningiomas commonly involve the cavernous sinus and produce ophthalmoplegia. Meningiomas are hypervascular tumors that derive their blood supply from the dural vessels. They also induce an osteoblastic reaction in the adjacent bone, resulting in a characteristic focal hyperostosis, which is well depicted on plain radiographs and CT. MRI signal is isointense relative to the brain on T1- and T2-weighted images. MRI is particularly good for assessing encasement of the cavernous sinus and carotid artery. The tumor is intensely enhancing on contrast-enhanced images.
Schwannomas
Schwannomas are nerve-sheath tumors that may involve cranial nerves III-XII or peripheral nerves. Schwannomas in the parasellar region arise from the trigeminal nerve and, in rare cases, from the third, fourth, or sixth cranial nerves. These tumors are benign, well encapsulated, and globular; all of these features that distinguish them from broad-based meningiomas. The tumors are isointense on T1-weighted MRIs and mildly hyperintense on T2-weighted MRIs. Cystic degeneration is frequent; hemorrhage and calcification are rare. The solid portions of these tumors are strongly enhancing with the use of intravenous contrast material.
Primary and secondary tumors of the skull base
A wide variety of primary and metastatic tumors of the skull base can involve the parasellar region. Nasopharyngeal carcinomas and malignant tumors of the paranasal sinuses may invade the parasellar region, and metastases from distant primary tumors may also involve the sphenoid bone and the parasellar region. The primary tumors include chordoma, chondroma, chondrosarcoma, and plasmacytoma.
Chordomas have uniform high signal intensity on T2-weighted MRIs. Chondrosarcomas are heterogeneous on images obtained with all sequences because of their variable calcification and chondral elements, but they usually have areas of high T2 signal intensity. Chordomas characteristically arise in the midline, whereas chondrosarcomas arise off midline at the synchondroses. Alteration of the normal hyperintense clival fat is a sensitive indicator of these tumors; therefore, nonenhanced non–fat-saturated T1-weighted imaging is particularly useful. Contrast enhancement is helpful in assessing the intracranial components of these tumors.
Carotid-cavernous fistula
The fistulous communication between the carotid artery and cavernous sinus may result from a dural arteriovenous malformation, trauma, or transsphenoidal surgery. Arterial pressures cause expansion of the cavernous sinus and dilation of parasellar and orbital veins. Proptosis, chemosis, and visual loss may complicate such fistulas. Flow effects and flow artifacts on MRI may confirm the diagnosis.
Granulomatous hypophysitis
Granulomatous hypophysitis is a rare entity that mimics a pituitary adenoma both clinically and radiologically. The reported causes of granulomatous hypophysitis include tuberculosis, syphilis, sarcoidosis, mycotic granuloma, and foreign body granuloma due to a ruptured Rathke cleft cyst. Lymphocytic hypophysitis represent inflammation of the pituitary gland and may complicate pregnancy or the postpartum period. MRI findings include diffuse enlargement of the anterior lobe and pituitary stalk. The gland is strongly enhanced with gadolinium-based contrast agents, and the enhancement commonly extends along the diaphragma sellae.
McCune-Albright syndrome
McCune-Albright syndrome (MAS) may clinically mimic acromegaly or gigantism. The syndrome is characterized by polyostotic fibrous dysplasia, café au lait pigmentation of the skin, and autonomous endocrine hyperfunction. The most common form of autonomous endocrine hyperfunction in this syndrome is gonadotropin-independent precocious puberty; however, affected individuals also may have hyperthyroidism, hypercortisolism, pituitary gigantism, or acromegaly. Nonendocrine abnormalities in this disorder include hypophosphatemia, chronic liver disease, tachycardia, and sudden death (which is rare and possibly due to cardiac arrhythmias).
Preferred examination
The clinical diagnosis of pituitary adenomas depends on the combination of symptoms and signs resulting from the size of the tumor and/or the type of hormone produced. CT and MRI have largely replaced plain radiography because conventional radiography is poor for delineating soft tissues.[1, 2, 3, 4, 5, 6, 7]Angiography is seldom performed; if indicated, CT angiography (CTA) and MRA have largely replaced conventional angiography. Angiography has a role when intervention is indicated in the cavernous sinus or in the cavernous part of the carotid artery.
Conventional single-section CT has a limited role in pituitary imaging, with a sensitivity of 17-22% in detecting microadenomas. Multidetector-row CT with 64 channels may have a role, especially in patients unable to undergo MRI. CT is best for visualizing bony detail and calcification in tumors such as germinomas, craniopharyngiomas, and meningiomas. CTA is excellent for showing the morphology of parasellar aneurysms and for presurgical planning. CT scans are valuable when MRI is contraindicated, as in patients with pacemakers or metallic implants in the brain or eyes.
MRI is generally preferred over CT for the diagnosis of pituitary adenomas because of its superior definition of small lesions in the pituitary sella and its improved anatomic definition before surgery. MRI is also preferred for postsurgical surveillance.
Somatostatin-receptor scintigraphy may be used to distinguish recurrent or residual tumor from scar or necrotic tissue after surgery.
Limitations of techniques
Conventional radiographs are poor for delineating soft tissues. MRI is more expensive than CT, but it is the preferred imaging study for the pituitary because it improves visualization of the soft tissues and vascular structures. Other limitations of CT include suboptimal imaging of the soft tissues compared with MRI, the need for intravenous contrast medium to enhance images, and the exposure to radiation.A potential pitfall of MRI diagnosis is a pneumatized anterior clinoid or calcification, which can simulate the flow void of an aneurysm. In addition, MRI is contraindicated in patients with pacemakers or ferromagnetic implants in the brain or eyes. With CT or MRI, pituitary adenoma remnants can be hard to differentiate from radiotherapy-induced fibrosis, especially in patients with clinically nonfunctioning pituitary adenomas, who lack circulating markers that aid in monitoring the progression or cure of the disease.
Brain Anatomy
To contact us Click HERE
The brain is composed of 3 main structural divisions: the cerebrum, the brainstem, and the cerebellum (see the images below). At the base of the brain is the brainstem, which extends from the upper cervical spinal cord to the diencephalon of the cerebrum. The brainstem is divided into the medulla, pons, and midbrain. Posterior to the brainstem lies the cerebellum.
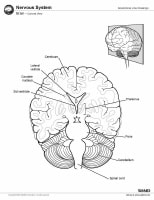 Brain, coronal view.
Brain, coronal view. 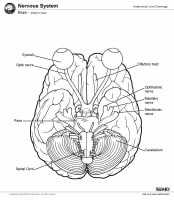 Brain, inferior view.
Brain, inferior view. 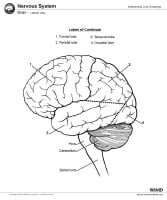 Brain, lateral view.
Brain, lateral view. 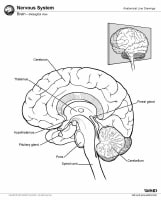 Brain, midsagittal view.
Brain, midsagittal view.
Each of the cerebral hemispheres is further divided into 4 lobes: the frontal lobe, the parietal lobe, the temporal lobe, and the occipital lobe. The medial temporal lobe structures are considered by some to be part of the so-called limbic lobe.
Briefly, the frontal lobe is distinguished from the parietal lobe posteriorly by the central sulcus (see the image below). The frontal lobe and parietal lobes are divided inferiorly from the temporal lobe by the lateral sulcus. The parietal lobe is distinguished from the occipital lobe by the parieto-occipital sulcus on the medial surface.
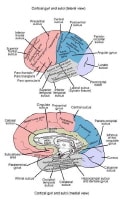 Lateral and medial surfaces of cerebrum, showing major sulci and gyri. The cerebrum is further divided into the telencephalon and diencephalon. The telencephalon consists of the cortex, the subcortical fibers, and the basal nuclei. The diencephalon mainly consists of the thalamus and hypothalamus. The telencephalon of the cerebrum is disproportionately well-developed in humans as compared with other mammals.
Lateral and medial surfaces of cerebrum, showing major sulci and gyri. The cerebrum is further divided into the telencephalon and diencephalon. The telencephalon consists of the cortex, the subcortical fibers, and the basal nuclei. The diencephalon mainly consists of the thalamus and hypothalamus. The telencephalon of the cerebrum is disproportionately well-developed in humans as compared with other mammals.
Below the cortex are axons, which are long fibers that emanate from and connect neurons. Axons are insulated by myelin, which increases the speed of conduction. Myelin is what gives the white appearance to these fibers of the brain--hence the term "white matter."
Limbic system
The limbic system is a grouping of cortical and subcortical structures involved in memory formation and emotional responses. The limbic system allows for complex interactions between the cortex, the thalamus, the hypothalamus, and the brainstem. The limbic system is not defined by strict anatomic boundaries but incorporates several important structures. The limbic structures conventionally include the amygdala, the hippocampus, the fornix, the mammillary bodies, the cingulate gyrus, and the parahippocampal gyrus.
The functional connections within the limbic system are best summarized by the Papez circuit. From the hippocampus, signals are relayed via the fornix to the mammillary bodies and via the mammillothalamic tract to the anterior nucleus of the thalamus. The thalamocingulate radiation then projects to the cingulate gyrus and back to the hippocampus to complete the circuit. The hippocampus serves as a primary output structure of the limbic system.
Unlike the 6-layered neocortex, the hippocampus only has 3 layers and is termed the archicortex. The hippocampus is felt to be a structure that is crucial to formation of memory--more specifically, a type of memory called declarative or explicit memory. Declarative memory is essentially the ability to recall life events of the past such as what meal was eaten for breakfast or where the car is parked.
Over time, however, certain declarative memories from the distant past can be independently recalled without the hippocampal structures. The hippocampus likely allows long-term memory encoding in the cortex and allows short-term memory retrieval. In laboratory studies of animals and humans, the hippocampus has been shown to also have a cellular memory termed "long-term potentiation."
The amygdala is a collection of nuclei that lies within the uncus. It receives multiple modes of sensory information as inputs. The outputs from the amygdala travel through the stria terminalis and the ventral amygdalofugal pathway. Output structures include the hypothalamus, as well as the thalamus, hippocampus, brainstem, and cortex. The amygdala appears to be involved in mediating the emotional aspects of memory, especially the subjective aspects of fear responses.
The primary input to the basal nuclei is from the primary motor cortex and premotor cortex (Brodmann areas 4 and 6) and consists primarily of the pyramidal cells in cortical layer V. These excitatory projections lead primarily to the striatum. The striatum also receives input from the dopaminergic cells of the substantia nigra. In turn, the striatum sends inhibitory projections to the globus pallidus externa and interna. The globus pallidus externa sends inhibitory projections to the subthalamic nucleus, which sends excitatory projections to the globus pallidus interna. The globus pallidus interna in turn projects to the ventral anterior and ventral lateral nuclei of the thalamus.
Certain movement disorders can be traced to pathologies in the basal nuclei, the most notable being Parkinson disease, which is related to deficiencies of dopaminergic cells of the substantia nigra. Huntington disease is a heritable disorder that involves degeneration of the striatum and leads to progressive jerky, or choreiform, movement.
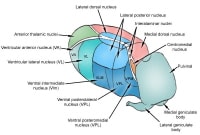 Major nuclei of thalamus. Left and right sides of the thalamus are divided by the third ventricle. Each side is then divided by the internal medullary lamina into a series of anterior nuclei, ventrolateral nuclei, and medial nuclei. Smaller nuclei are found within these regions, numbering perhaps in excess of 100.
Major nuclei of thalamus. Left and right sides of the thalamus are divided by the third ventricle. Each side is then divided by the internal medullary lamina into a series of anterior nuclei, ventrolateral nuclei, and medial nuclei. Smaller nuclei are found within these regions, numbering perhaps in excess of 100.
The anterior thalamic nuclei are functionally associated with the limbic system and share reciprocal connections with the cingulate gyrus and the mammillary bodies. The medial nuclei project to the frontal association cortex and premotor cortex, with reciprocal connectivity.
The ventrolateral nuclei can be further divided into the ventral anterior (VA), ventral lateral (VL), ventral posterolateral (VPL), and ventral posteromedial (VPM) nuclei. The VA and VL nuclei share input from the globus pallidus and projections to the motor cortex. The VPL and VPM serve as sensory relays in the body and face, respectively.
The lateral nuclei are divided into lateral dorsal and lateral posterior nuclei, with projections to the cingulate gyrus and parietal cortex, respectively.
Other thalamic structures not included in the anatomic divisions above include the medial and lateral geniculate bodies, which process auditory and visual information, respectively. The pulvinar connects reciprocally with the parietal and occipital association cortex. Intralaminar nuclei within the internal medullary lamina obtain input from the brainstem, cerebellum, and other thalamic nuclei and project to basal nuclei structures and other thalamic nuclei. Amongst the intralaminar nuclei, the centromedian nucleus is a part of the reticular activating system, which plays a role in maintaining cortical arousal.
The anterior nuclei include the preoptic, the supraoptic, and paraventricular nuclei. The posterior nuclei include the supramammillary nucleus, the mammillary nucleus, the intercalate nucleus, and the posterior nucleus. The middle nuclei include the infundibular, tuberal, dorsomedial, ventromedial, and lateral nuclei.
Parasympathetic control can be attributed to the anterior and medial nuclear groups, whereas sympathetic control can be attributed to the posterior and lateral nuclear groups. Satiety can be localized to stimulation of medial nuclei, and hunger can be localized to stimulation of lateral nuclei. Other functions of the hypothalamus include regulation of body temperature, heart rate, blood pressure, and water balance.
The hypothalamus has close connections with the cingulate gyrus, frontal lobe, hippocampus, thalamus, brainstem, spinal cord, basal ganglia, and pituitary gland.
The left and right cerebral hemispheres are separated by the longitudinal cerebral fissure. The principal connection between the 2 hemispheres is the corpus callosum. Each cortical hemisphere can be divided into 4 lobes: frontal, temporal, parietal, and occipital. The frontal lobe can be distinguished from the temporal lobe by the lateral sulcus (Sylvian fissure). The frontal lobe can be distinguished from the parietal lobe by the central sulcus (Rolandic fissure). The parieto-occipital fissure, which is visible on the medial aspect of the hemisphere, divides the parietal and occipital lobes. Within the lateral sulcus is another cortical surface referred to as the insula.
The frontal lobe can then be further divided into the superior, middle, and inferior frontal gyri, which are divided by the superior and inferior frontal sulci, respectively. The inferior frontal gyrus forms the frontal operculum, which overlies the lateral sulcus. The frontal operculum can be divided into 3 triangular gyri: the pars orbitalis, the pars triangularis, and the pars opercularis, in order from anterior to posterior. The precentral gyrus is the gyrus immediately anterior to the central sulcus.
Similarly, the temporal lobe is divided into the superior, middle, and inferior temporal gyri, which are separated by the superior and inferior temporal sulci. On the inferior surface of the temporal lobe just lateral to the midbrain the parahippocampal gyrus can be identified, with the collateral sulcus lying lateral. Between the parahippocampal gyrus and the inferior temporal gyrus lies the occipitotemporal gyrus, also known as the fusiform gyrus.
Within the parietal lobe, the superior temporal sulcus is capped by the angular gyrus. Just above this, the lateral sulcus is capped by the supramarginal gyrus. Just below the angular gyrus, the lateral occipital gyrus caps the inferior temporal sulcus.
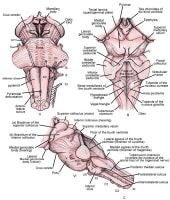 Three views of brainstem.
Three views of brainstem.
Dorsally, 2 pairs of protrusions are visible, which are the gracile tubercles medially and the cuneate tubercles just lateral to those. These represent the nuclei where sensory information from the dorsal columns is relayed onto thalamic projection neurons. Just superior to these protrusions is the floor of the fourth ventricle, which bears several characteristic impressions. The vagal trigone is the dorsal nucleus of the vagal nerve and lies inferiorly, just below the hypoglossal trigone.
On either side of the midline, there are bulges that are produced by the descending corticospinal tracts. At the pontomedullary junction, the 6th cranial nerve can be seen exiting the brainstem. Laterally, but anterior to the middle cerebellar peduncle, the fifth cranial nerve is seen exiting the brainstem. Below the middle cerebellar peduncle, the seventh and eighth cranial nerves can be seen exiting. Dorsally, the pons forms the floor of the fourth ventricle.
The posterior aspect of the midbrain has 2 pairs of characteristic protrusions, the superior and inferior colliculi. The superior colliculi are involved in mediating the vestibulo-ocular reflex, whereas the inferior colliculi are involved in sound localization.
The cerebellum consists of 2 hemispheres, connected by a midline structure called the vermis. In contrast to the neocortex of the cerebrum, the cerebellar cortex has 3 layers: molecular, Purkinje, and granular. There are 4 deep cerebellar nuclei: the fastigial, globose, emboliform, and dentate nuclei, in sequence from medial to lateral. The afferent and efferent pathways to and from the cerebellum exist within the 3 cerebellar peduncles.
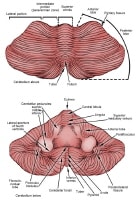 Top and anterior views of cerebellum. In children, the cerebellum is a common location for tumors such as juvenile pilocytic astrocytomas and medulloblastomas. In adults, the posterior fossa is a very common location for metastatic tumors but also a common location for tumors such as hemangioblastomas. Another pathology of the posterior fossa can occur when the cerebellar tonsils descend below the foramen magnum; this is termed a Chiari I malformation.
Top and anterior views of cerebellum. In children, the cerebellum is a common location for tumors such as juvenile pilocytic astrocytomas and medulloblastomas. In adults, the posterior fossa is a very common location for metastatic tumors but also a common location for tumors such as hemangioblastomas. Another pathology of the posterior fossa can occur when the cerebellar tonsils descend below the foramen magnum; this is termed a Chiari I malformation.
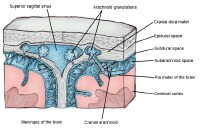 Cross-sectional view of meninges and dural venous sinus. The innermost of the 3 layers is the pia mater, which tightly covers the brain itself, conforming to its grooves and folds. This layer is rich with blood vessels that descend into the brain.
Cross-sectional view of meninges and dural venous sinus. The innermost of the 3 layers is the pia mater, which tightly covers the brain itself, conforming to its grooves and folds. This layer is rich with blood vessels that descend into the brain.
Outside the pia mater, which tightly contours the brain, is the arachnoid mater. The arachnoid mater is a thin weblike layer. Between the pia mater and the arachnoid mater is a space called the subarachnoid space, which contains cerebrospinal fluid (CSF). This space is where the major blood arteries supplying blood to the brain lie. If a blood vessel ruptures in this space, it can cause a subarachnoid hemorrhage. The arachnoid cap cells can give rise to meningiomas, a usually benign tumor.
The outermost meningeal layer is the dura mater, which lines the interior of the skull. The dura mater is composed of 2 individual layers, the meningeal dura and the periosteal dura. For the most part, these layers are fused; venous sinuses can be found in areas of separation. The tentorium cerebelli is a dura mater fold that separates the cerebellum from the cerebrum. The falx cerebri is a fold that separates the left and right cerebral hemispheres.
Between the arachnoid mater and the dura mater is the subdural space. If bleeding occurs in the space underneath the dura mater, it is called a subdural hematoma. If bleeding occurs outside the dura but underneath the skull, this is called an epidural hematoma.
The brain has 4 ventricles (see the image below). Within the cerebral hemispheres are the lateral ventricles, which are connected to each other and to the third ventricle through a pathway called the interventricular foramen (of Monro). The third ventricle lies in the midline, separating deeper brain structures such as the left and right thalami. The third ventricle communicates with the fourth ventricle through the cerebral aqueduct (of Sylvius), which is a long narrow tube.
 Ventricular system, which circulates cerebrospinal fluid through brain. From the fourth ventricle, CSF flows into the subarachnoid space around both the brain and the spinal cord. From the subarachnoid space, which lines the cerebral hemispheres, CSF is then absorbed into the venous circulatory system. Arachnoid villi are structures within the superior sagittal sinus that release CSF back into the venous system.
Ventricular system, which circulates cerebrospinal fluid through brain. From the fourth ventricle, CSF flows into the subarachnoid space around both the brain and the spinal cord. From the subarachnoid space, which lines the cerebral hemispheres, CSF is then absorbed into the venous circulatory system. Arachnoid villi are structures within the superior sagittal sinus that release CSF back into the venous system.
Hydrocephalus is a condition in which production of CSF is disproportionate to absorption. This is most commonly caused by impaired absorption resulting from obstruction of the CSF pathways, in which case it is termed obstructive hydrocephalus. This also occurs when the absorption of CSF is impaired, in which case it is termed communicating hydrocephalus. Rarely is hydrocephalus caused by increased CSF production.
The basilar artery, the posterior cerebral arteries, the posterior communicating arteries, and the anterior cerebral arteries, along with the anterior communication artery, form an important collateral circulation at the base of the brain termed the cerebral arterial circle (of Willis). These vessels lie within the subarachnoid space and are a common location for cerebral aneurysms to form.
Venous return to the heart occurs through a combination of deep cerebral veins and superficial cortical veins. The veins then contribute to larger venous sinuses, which lie within the dura and ultimately drain through the internal jugular veins to the brachiocephalic veins and then into the superior vena cava.
When examined histologically, the neurons of the cortical gray matter demonstrate a laminar pattern. The neocortex contains 6 distinct layers, in contrast to the evolutionarily older paleocortex and archicortex, which typically contain 3 layers. The specific cytoarchitectural patterns of the cortex are not uniform throughout the cerebral cortex, and their variation was mapped by the German physician Korbinian Brodmann and presented in 1909. The so-called Brodmann areas represent cytoarchitectural differences across different brain regions, and the numbering scheme developed by Brodmann is still used to refer to distinct areas of the cortex.[4]
Projection fibers connect structures over the longest distances, such as the corticospinal projections from the motor cortex to the anterior horn cells of the spinal cord. Association fibers connect structures within the same hemisphere, such as the arcuate fasciculus, which connects the temporoparietal receptive speech areas with the frontal speech areas. Commissural fibers connect homologous structures in the left and right hemispheres, the most notable example being the corpus callosum.
Diffusion tensor imaging has emerged recently as a magnetic resonance imaging tool that provides exceptionally detailed white matter tractography in both normal and pathologic anatomy.
Microglia have a function in the brain similar to that of the immune system. Astrocytes play a role in creating the blood-brain barrier, which allows certain substances to selectively pass from the capillary system. They are also responsible for reactive scar formation in the brain. Oligodendrocytes form myelin, which serves to electrically insulate the axons of nerve cells, allowing increased rates of conduction.
Abnormal proliferation of oligodendrocytes and astrocytes can lead to primary brain tumors called oligodendrogliomas and astrocytomas. Collectively, these belong to a family of tumors called gliomas, and the most aggressive type is termed a glioblastoma multiforme.
 Functional localization within cerebral cortex. Some of the earliest contributions to modern language mapping can be traced to the work of neurologist Paul Broca, who studied the language deficits in patients with stroke. Broca's area, as it is termed, is a region of the frontal operculum, which also overlaps with Brodmann area 44 and 45. Three overlapping names describe this region, which is responsible for speech production. Selective damage to this region leads to difficulty speaking but typically with preserved comprehension.
Functional localization within cerebral cortex. Some of the earliest contributions to modern language mapping can be traced to the work of neurologist Paul Broca, who studied the language deficits in patients with stroke. Broca's area, as it is termed, is a region of the frontal operculum, which also overlaps with Brodmann area 44 and 45. Three overlapping names describe this region, which is responsible for speech production. Selective damage to this region leads to difficulty speaking but typically with preserved comprehension.
In contrast, Wernicke's area refers to the posterior aspect of the superior temporal gyrus, which overlaps with Brodmann area 22. This region is generally responsible for speech comprehension, and selective injury to it can lead to impaired understanding with preserved speech production.
Additionally, language function is hemispherically dominant. This means that Broca's and Wernicke's aphasia typically result from damage to the hemisphere that is dominant for language. In right-handed individuals, the left hemisphere is nearly always dominant for language. However, among left-handed individuals, the left hemisphere is dominant for speech in only 70%. Bilateral representation occurs in 15% of left-handed people, and right-hemisphere language representation occurs in 15% of left-handed people.
The primary motor and sensory cortex have been mapped extensively through intraoperative stimulation in awake patients. Early work performed by neurosurgeon Wilder Penfield in Montreal led to the conceptualization of the homunculus, which is the somatotopic representation of the body in both the primary motor and primary sensory cortex (see the image below).
 Homunculus is somatotopic representation of human body in primary motor and sensory cortex. Some body parts are disproportionately represented because there are more motor and sensory fibers devoted to regions such as face and fingers. The primary motor cortex corresponds with the precentral gyrus, or Brodmann area 4. Intraoperative stimulation of the motor cortex in awake patients leads to contralateral muscle contraction in a single muscle or discrete group of muscles. The premotor cortex, which corresponds to Brodmann area 6, is also occupied with movement, but typically more complex movements are elicited by stimulation here.
Homunculus is somatotopic representation of human body in primary motor and sensory cortex. Some body parts are disproportionately represented because there are more motor and sensory fibers devoted to regions such as face and fingers. The primary motor cortex corresponds with the precentral gyrus, or Brodmann area 4. Intraoperative stimulation of the motor cortex in awake patients leads to contralateral muscle contraction in a single muscle or discrete group of muscles. The premotor cortex, which corresponds to Brodmann area 6, is also occupied with movement, but typically more complex movements are elicited by stimulation here.
The primary sensory cortex corresponds with the postcentral gyrus, or Brodmann areas 1-3. The homunculus obtained from awake mapping corresponds to that of the motor cortex. Stimulation in awake patients during surgery typically leads to the subjective sensation of tingling of the corresponding body part on the opposite side of the body. Caudally, the superior parietal lobule, Brodmann areas 5 and 7, represents the secondary sensory cortex, which is felt to subserve multimodal sensory information.
The primary visual cortex corresponds to Brodmann area 17 and occupies the occipital pole. It is also termed the striate cortex. The visual cortex is retinotopically organized. Surrounding the primary visual cortex is the visual association cortex, or Brodmann areas 18 and 19.
The primary auditory cortex lies on the superior bank of the superior temporal gyrus and corresponds to Brodmann area 41. Like the primary motor, primary sensory, and visual cortices, the primary auditory cortex is tonotopically organized. The auditory association cortex, or Brodmann area 42, surrounds the primary auditory cortex.
Overview
The central nervous system consists of the brain and the spinal cord. The peripheral nervous system consists of the extensions of neural structures beyond the dural lining of the central nervous system and includes somatic and autonomic divisions.The brain is composed of 3 main structural divisions: the cerebrum, the brainstem, and the cerebellum (see the images below). At the base of the brain is the brainstem, which extends from the upper cervical spinal cord to the diencephalon of the cerebrum. The brainstem is divided into the medulla, pons, and midbrain. Posterior to the brainstem lies the cerebellum.
 Brain, coronal view.
Brain, coronal view.  Brain, inferior view.
Brain, inferior view.  Brain, lateral view.
Brain, lateral view.  Brain, midsagittal view.
Brain, midsagittal view. Gross Anatomy: Cerebrum
The cerebrum is the largest component of the brain. It is divided into right and left hemispheres. The corpus callosum is the collection of white matter fibers that joins these hemispheres.Each of the cerebral hemispheres is further divided into 4 lobes: the frontal lobe, the parietal lobe, the temporal lobe, and the occipital lobe. The medial temporal lobe structures are considered by some to be part of the so-called limbic lobe.
Briefly, the frontal lobe is distinguished from the parietal lobe posteriorly by the central sulcus (see the image below). The frontal lobe and parietal lobes are divided inferiorly from the temporal lobe by the lateral sulcus. The parietal lobe is distinguished from the occipital lobe by the parieto-occipital sulcus on the medial surface.
 Lateral and medial surfaces of cerebrum, showing major sulci and gyri. The cerebrum is further divided into the telencephalon and diencephalon. The telencephalon consists of the cortex, the subcortical fibers, and the basal nuclei. The diencephalon mainly consists of the thalamus and hypothalamus. The telencephalon of the cerebrum is disproportionately well-developed in humans as compared with other mammals.
Lateral and medial surfaces of cerebrum, showing major sulci and gyri. The cerebrum is further divided into the telencephalon and diencephalon. The telencephalon consists of the cortex, the subcortical fibers, and the basal nuclei. The diencephalon mainly consists of the thalamus and hypothalamus. The telencephalon of the cerebrum is disproportionately well-developed in humans as compared with other mammals. Cortex and subcortical fibers
The outermost layer of the cerebrum is the cortex, which has a slightly gray appearance--hence the term "gray matter." The cortex has a folded structure; each fold is termed a gyrus, while each groove between the folds is termed a sulcus. Cortical anatomy is discussed in greater detail below.Below the cortex are axons, which are long fibers that emanate from and connect neurons. Axons are insulated by myelin, which increases the speed of conduction. Myelin is what gives the white appearance to these fibers of the brain--hence the term "white matter."
Limbic system
The limbic system is a grouping of cortical and subcortical structures involved in memory formation and emotional responses. The limbic system allows for complex interactions between the cortex, the thalamus, the hypothalamus, and the brainstem. The limbic system is not defined by strict anatomic boundaries but incorporates several important structures. The limbic structures conventionally include the amygdala, the hippocampus, the fornix, the mammillary bodies, the cingulate gyrus, and the parahippocampal gyrus.
The functional connections within the limbic system are best summarized by the Papez circuit. From the hippocampus, signals are relayed via the fornix to the mammillary bodies and via the mammillothalamic tract to the anterior nucleus of the thalamus. The thalamocingulate radiation then projects to the cingulate gyrus and back to the hippocampus to complete the circuit. The hippocampus serves as a primary output structure of the limbic system.
Unlike the 6-layered neocortex, the hippocampus only has 3 layers and is termed the archicortex. The hippocampus is felt to be a structure that is crucial to formation of memory--more specifically, a type of memory called declarative or explicit memory. Declarative memory is essentially the ability to recall life events of the past such as what meal was eaten for breakfast or where the car is parked.
Over time, however, certain declarative memories from the distant past can be independently recalled without the hippocampal structures. The hippocampus likely allows long-term memory encoding in the cortex and allows short-term memory retrieval. In laboratory studies of animals and humans, the hippocampus has been shown to also have a cellular memory termed "long-term potentiation."
The amygdala is a collection of nuclei that lies within the uncus. It receives multiple modes of sensory information as inputs. The outputs from the amygdala travel through the stria terminalis and the ventral amygdalofugal pathway. Output structures include the hypothalamus, as well as the thalamus, hippocampus, brainstem, and cortex. The amygdala appears to be involved in mediating the emotional aspects of memory, especially the subjective aspects of fear responses.
Basal nuclei (ganglia)
The basal nuclei (formerly referred to as the basal ganglia) comprise the caudate nucleus, putamen, globus pallidus, subthalamic nucleus, and substantia nigra. Pairs of these structures bear different names. The putamen and globus pallidus combined form the lentiform nuclei. The putamen and caudate nucleus combined form the striatum. The striatum derives its name from the striped appearance given by the gray matter connections bridging across the internal capsule. The basal nuclei are closely integrated with the motor cortex, premotor cortex, and motor nuclei of the thalamus and plays a crucial role in modulation of movements.The primary input to the basal nuclei is from the primary motor cortex and premotor cortex (Brodmann areas 4 and 6) and consists primarily of the pyramidal cells in cortical layer V. These excitatory projections lead primarily to the striatum. The striatum also receives input from the dopaminergic cells of the substantia nigra. In turn, the striatum sends inhibitory projections to the globus pallidus externa and interna. The globus pallidus externa sends inhibitory projections to the subthalamic nucleus, which sends excitatory projections to the globus pallidus interna. The globus pallidus interna in turn projects to the ventral anterior and ventral lateral nuclei of the thalamus.
Certain movement disorders can be traced to pathologies in the basal nuclei, the most notable being Parkinson disease, which is related to deficiencies of dopaminergic cells of the substantia nigra. Huntington disease is a heritable disorder that involves degeneration of the striatum and leads to progressive jerky, or choreiform, movement.
Thalamus
Positioned between the brainstem and the telencephalon, the diencephalon is composed of the thalamus, the epithalamus, the subthalamus, and the hypothalamus. The thalamus serves as a relay station for ascending input to the cortex and receives information from each of the cardinal senses (except smell). It is hypothesized that the thalamus serves a gating function in filtering information. The thalamus consists of multiple nuclei that are briefly described here (see the image below). Major nuclei of thalamus. Left and right sides of the thalamus are divided by the third ventricle. Each side is then divided by the internal medullary lamina into a series of anterior nuclei, ventrolateral nuclei, and medial nuclei. Smaller nuclei are found within these regions, numbering perhaps in excess of 100.
Major nuclei of thalamus. Left and right sides of the thalamus are divided by the third ventricle. Each side is then divided by the internal medullary lamina into a series of anterior nuclei, ventrolateral nuclei, and medial nuclei. Smaller nuclei are found within these regions, numbering perhaps in excess of 100. The anterior thalamic nuclei are functionally associated with the limbic system and share reciprocal connections with the cingulate gyrus and the mammillary bodies. The medial nuclei project to the frontal association cortex and premotor cortex, with reciprocal connectivity.
The ventrolateral nuclei can be further divided into the ventral anterior (VA), ventral lateral (VL), ventral posterolateral (VPL), and ventral posteromedial (VPM) nuclei. The VA and VL nuclei share input from the globus pallidus and projections to the motor cortex. The VPL and VPM serve as sensory relays in the body and face, respectively.
The lateral nuclei are divided into lateral dorsal and lateral posterior nuclei, with projections to the cingulate gyrus and parietal cortex, respectively.
Other thalamic structures not included in the anatomic divisions above include the medial and lateral geniculate bodies, which process auditory and visual information, respectively. The pulvinar connects reciprocally with the parietal and occipital association cortex. Intralaminar nuclei within the internal medullary lamina obtain input from the brainstem, cerebellum, and other thalamic nuclei and project to basal nuclei structures and other thalamic nuclei. Amongst the intralaminar nuclei, the centromedian nucleus is a part of the reticular activating system, which plays a role in maintaining cortical arousal.
Epithalamus
The epithalamus is made up of the habenula, the habenular commissure, the posterior commissure, and the pineal gland.Subthalamus
Located between the midbrain and the thalamus, the subthalamus contains the subthalamic nucleus, the red nucleus, and the substantia nigra. Subthalamic structures are closely integrated with the basal ganglia and play a role in modulation of movement.Hypothalamus
Thy hypothalamic nuclei lie in the walls of the third ventricle anteriorly. The hypothalamus is involved in mediating endocrine, autonomic, visceral, and homeostatic functions. It can roughly be divided into anterior, posterior, and middle groups of nuclei.The anterior nuclei include the preoptic, the supraoptic, and paraventricular nuclei. The posterior nuclei include the supramammillary nucleus, the mammillary nucleus, the intercalate nucleus, and the posterior nucleus. The middle nuclei include the infundibular, tuberal, dorsomedial, ventromedial, and lateral nuclei.
Parasympathetic control can be attributed to the anterior and medial nuclear groups, whereas sympathetic control can be attributed to the posterior and lateral nuclear groups. Satiety can be localized to stimulation of medial nuclei, and hunger can be localized to stimulation of lateral nuclei. Other functions of the hypothalamus include regulation of body temperature, heart rate, blood pressure, and water balance.
The hypothalamus has close connections with the cingulate gyrus, frontal lobe, hippocampus, thalamus, brainstem, spinal cord, basal ganglia, and pituitary gland.
Gross Anatomy: Cortex
The neocortex is the most phylogenetically developed structure of the human brain as compared with the brains of other species. The complex pattern of folding allows an increased cortical surface to occupy a smaller cranial volume. The pattern of folding that forms the sulcal and gyral patterns remains highly preserved across individuals. This enables a nomenclature for the cortical anatomy.The left and right cerebral hemispheres are separated by the longitudinal cerebral fissure. The principal connection between the 2 hemispheres is the corpus callosum. Each cortical hemisphere can be divided into 4 lobes: frontal, temporal, parietal, and occipital. The frontal lobe can be distinguished from the temporal lobe by the lateral sulcus (Sylvian fissure). The frontal lobe can be distinguished from the parietal lobe by the central sulcus (Rolandic fissure). The parieto-occipital fissure, which is visible on the medial aspect of the hemisphere, divides the parietal and occipital lobes. Within the lateral sulcus is another cortical surface referred to as the insula.
The frontal lobe can then be further divided into the superior, middle, and inferior frontal gyri, which are divided by the superior and inferior frontal sulci, respectively. The inferior frontal gyrus forms the frontal operculum, which overlies the lateral sulcus. The frontal operculum can be divided into 3 triangular gyri: the pars orbitalis, the pars triangularis, and the pars opercularis, in order from anterior to posterior. The precentral gyrus is the gyrus immediately anterior to the central sulcus.
Similarly, the temporal lobe is divided into the superior, middle, and inferior temporal gyri, which are separated by the superior and inferior temporal sulci. On the inferior surface of the temporal lobe just lateral to the midbrain the parahippocampal gyrus can be identified, with the collateral sulcus lying lateral. Between the parahippocampal gyrus and the inferior temporal gyrus lies the occipitotemporal gyrus, also known as the fusiform gyrus.
Within the parietal lobe, the superior temporal sulcus is capped by the angular gyrus. Just above this, the lateral sulcus is capped by the supramarginal gyrus. Just below the angular gyrus, the lateral occipital gyrus caps the inferior temporal sulcus.
Gross Anatomy: Brainstem and Cranial Nerves
Evolutionarily, the brainstem is the most ancient part of the brain. Structurally, it can be divided into the medulla, pons, and midbrain. These three structures are briefly described below. Cross-sectional anatomy of the brainstem is rather complex, given the multiple traversing pathways and cranial nerve nuclei (see the image below).[1, 2, 3] Three views of brainstem.
Three views of brainstem. Medulla
The medulla is continuous with and superior to the cervical spinal cord. There are several external anatomic features of the medulla that can be visible grossly. Ventrally, the pyramids and pyramidal decussation is visualized just below the pons. These are the descending corticospinal tracts. Just lateral to the pyramids, the hypoglossal nerve can be seen as it exits the brainstem. Lateral to the hypoglossal nerve is the inferior olive. Dorsolateral to the inferior olive, the 9th, 10th and 11th cranial nerves exit.Dorsally, 2 pairs of protrusions are visible, which are the gracile tubercles medially and the cuneate tubercles just lateral to those. These represent the nuclei where sensory information from the dorsal columns is relayed onto thalamic projection neurons. Just superior to these protrusions is the floor of the fourth ventricle, which bears several characteristic impressions. The vagal trigone is the dorsal nucleus of the vagal nerve and lies inferiorly, just below the hypoglossal trigone.
Pons
Superior to the medulla lies the pons, the ventral surface of which has a characteristic band of horizontal fibers. These fibers are the pontocerebellar fibers that are in turn projections from the corticopontine fibers. They cross to enter the contralateral middle cerebellar peduncle and thus enter the cerebellum.On either side of the midline, there are bulges that are produced by the descending corticospinal tracts. At the pontomedullary junction, the 6th cranial nerve can be seen exiting the brainstem. Laterally, but anterior to the middle cerebellar peduncle, the fifth cranial nerve is seen exiting the brainstem. Below the middle cerebellar peduncle, the seventh and eighth cranial nerves can be seen exiting. Dorsally, the pons forms the floor of the fourth ventricle.
Midbrain
The midbrain, also termed the mesencephalon, is the superiormost aspect of the brainstem. Ventrally, the midbrain appears as 2 bundles that diverge rostrally as the cerebral peduncles. Between the cerebral peduncles, the third cranial nerve can be seen exiting. The fourth cranial nerve exits dorsally and is unique in this regard. They then course anteriorly around the cerebral peduncles.The posterior aspect of the midbrain has 2 pairs of characteristic protrusions, the superior and inferior colliculi. The superior colliculi are involved in mediating the vestibulo-ocular reflex, whereas the inferior colliculi are involved in sound localization.
Cranial nerves
There are 12 pairs of cranial nerves that function mainly to convey motor signals to and sensory information from the head and neck. The lower cranial nerves have somewhat more complex visceral functions that are not strictly limited to the head and neck. The cranial nerves are as follows:- I: The olfactory nerve relays information from the nerves of the olfactory epithelium to mesial temporal lobe and frontal lobe structures
- II: The optic nerve relays visual information from the retina; the right and left optic nerves then join at the optic chiasm, where they give rise to the optic tracts, which convey visual information to the thalamus and brainstem and, ultimately, the visual cortex; optic gliomas can arise from the optic nerve
- III: The oculomotor nerve is principally involved in the control of eye movements through its innervation of the superior rectus, the medial rectus, the inferior rectus, and the inferior oblique muscles
- IV: The trochlear nerve innervates the superior oblique muscle and is purely a motor nerve
- V: The trigeminal nerve is both a motor and sensory nerve and has 3 divisions, V1 (the ophthalmic division), V2 (the maxillary division), and V3 (the mandibular division); it is involved in conveying sensory information from the face and also in controlling the muscles of mastication; vascular compression of the branches of the trigeminal nerve near its entry into the brainstem has been associated with some types of facial pain, including trigeminal neuralgia
- VI: The abducens nerve innervates the lateral rectus nerve, allowing lateral eye movements
- VII: The facial nerve is principally involved in innervation of the muscles of facial expression and also plays a role in tearing, salivation, and taste; Bell's palsy is a relatively common facial nerve palsy
- VIII: The vestibulocochlear nerve is a purely sensory nerve that conveys auditory information from the cochlea to the brainstem via the cochlear branch; the vestibular branch conveys proprioceptive information about head position and movement from the inner ear to the brainstem; acoustic neuromas are typically benign tumors that can arise from the vestibular portion of this nerve
- IX: The glossopharyngeal nerve is involved in taste and salivation, as well as sensation in the oropharynx; the afferent limb of the gag reflex is mediated by the glossopharyngeal nerve
- X: The vagus nerve conveys visceral sensation to the brainstem and also controls some visceral functions, such as heart rate and gastrointestinal motility
- XI: The accessory nerve has contributions from a spinal component and innervates neck muscles involved in head turning
- XII: The hypoglossal nerve is a motor nerve that innervates muscles of the tongue
Gross Anatomy: Cerebellum
The cerebellum occupies the posterior fossa, dorsal to the pons and medulla. It is involved primarily in modulating motor control to enable precisely coordinated body movements. Similar to the cerebrum, which has gyri and sulci, the cerebellum has finer folia and fissures that increase the surface area.The cerebellum consists of 2 hemispheres, connected by a midline structure called the vermis. In contrast to the neocortex of the cerebrum, the cerebellar cortex has 3 layers: molecular, Purkinje, and granular. There are 4 deep cerebellar nuclei: the fastigial, globose, emboliform, and dentate nuclei, in sequence from medial to lateral. The afferent and efferent pathways to and from the cerebellum exist within the 3 cerebellar peduncles.
 Top and anterior views of cerebellum. In children, the cerebellum is a common location for tumors such as juvenile pilocytic astrocytomas and medulloblastomas. In adults, the posterior fossa is a very common location for metastatic tumors but also a common location for tumors such as hemangioblastomas. Another pathology of the posterior fossa can occur when the cerebellar tonsils descend below the foramen magnum; this is termed a Chiari I malformation.
Top and anterior views of cerebellum. In children, the cerebellum is a common location for tumors such as juvenile pilocytic astrocytomas and medulloblastomas. In adults, the posterior fossa is a very common location for metastatic tumors but also a common location for tumors such as hemangioblastomas. Another pathology of the posterior fossa can occur when the cerebellar tonsils descend below the foramen magnum; this is termed a Chiari I malformation. Gross Anatomy: Meninges
The meninges consist of 3 tissue layers that cover the brain and spinal cord: the pia, arachnoid, and the dura mater (see the image below). The pia along with the arachnoid are referred to as the leptomeninx, whereas the dura is referred to as the pachymeninx. Cross-sectional view of meninges and dural venous sinus. The innermost of the 3 layers is the pia mater, which tightly covers the brain itself, conforming to its grooves and folds. This layer is rich with blood vessels that descend into the brain.
Cross-sectional view of meninges and dural venous sinus. The innermost of the 3 layers is the pia mater, which tightly covers the brain itself, conforming to its grooves and folds. This layer is rich with blood vessels that descend into the brain.Outside the pia mater, which tightly contours the brain, is the arachnoid mater. The arachnoid mater is a thin weblike layer. Between the pia mater and the arachnoid mater is a space called the subarachnoid space, which contains cerebrospinal fluid (CSF). This space is where the major blood arteries supplying blood to the brain lie. If a blood vessel ruptures in this space, it can cause a subarachnoid hemorrhage. The arachnoid cap cells can give rise to meningiomas, a usually benign tumor.
The outermost meningeal layer is the dura mater, which lines the interior of the skull. The dura mater is composed of 2 individual layers, the meningeal dura and the periosteal dura. For the most part, these layers are fused; venous sinuses can be found in areas of separation. The tentorium cerebelli is a dura mater fold that separates the cerebellum from the cerebrum. The falx cerebri is a fold that separates the left and right cerebral hemispheres.
Between the arachnoid mater and the dura mater is the subdural space. If bleeding occurs in the space underneath the dura mater, it is called a subdural hematoma. If bleeding occurs outside the dura but underneath the skull, this is called an epidural hematoma.
Gross Anatomy: Ventricles and Cerebrospinal Fluid
The brain is bathed in cerebrospinal fluid (CSF), which is continuously produced and absorbed. The ventricles are CSF-containing cavities within the brain. The structures that produce CSF are contained within the ventricles and are called the choroid plexus. CSF is produced at a rate of about 450 mL/day, although at any given time about 150 mL can be found within the CSF spaces. Thus, the volume of CSF in most adults is turned over about 3 times per day.The brain has 4 ventricles (see the image below). Within the cerebral hemispheres are the lateral ventricles, which are connected to each other and to the third ventricle through a pathway called the interventricular foramen (of Monro). The third ventricle lies in the midline, separating deeper brain structures such as the left and right thalami. The third ventricle communicates with the fourth ventricle through the cerebral aqueduct (of Sylvius), which is a long narrow tube.
 Ventricular system, which circulates cerebrospinal fluid through brain. From the fourth ventricle, CSF flows into the subarachnoid space around both the brain and the spinal cord. From the subarachnoid space, which lines the cerebral hemispheres, CSF is then absorbed into the venous circulatory system. Arachnoid villi are structures within the superior sagittal sinus that release CSF back into the venous system.
Ventricular system, which circulates cerebrospinal fluid through brain. From the fourth ventricle, CSF flows into the subarachnoid space around both the brain and the spinal cord. From the subarachnoid space, which lines the cerebral hemispheres, CSF is then absorbed into the venous circulatory system. Arachnoid villi are structures within the superior sagittal sinus that release CSF back into the venous system.Hydrocephalus is a condition in which production of CSF is disproportionate to absorption. This is most commonly caused by impaired absorption resulting from obstruction of the CSF pathways, in which case it is termed obstructive hydrocephalus. This also occurs when the absorption of CSF is impaired, in which case it is termed communicating hydrocephalus. Rarely is hydrocephalus caused by increased CSF production.
Gross Anatomy: Blood Vessels
Arteries supply blood to the brain via 2 main pairs of vessels: the internal carotid artery and the vertebral artery on each side. The internal carotid artery on each side terminates into the anterior cerebral artery, the middle cerebral artery, and the posterior communicating artery. The vertebral arteries on each side join to form the basilar artery. The basilar artery then gives rise to the posterior cerebral arteries and the superior cerebellar arteries.The basilar artery, the posterior cerebral arteries, the posterior communicating arteries, and the anterior cerebral arteries, along with the anterior communication artery, form an important collateral circulation at the base of the brain termed the cerebral arterial circle (of Willis). These vessels lie within the subarachnoid space and are a common location for cerebral aneurysms to form.
Venous return to the heart occurs through a combination of deep cerebral veins and superficial cortical veins. The veins then contribute to larger venous sinuses, which lie within the dura and ultimately drain through the internal jugular veins to the brachiocephalic veins and then into the superior vena cava.
Microscopic Anatomy
The cellular structure of the brain is composed primarily of neurons and their support cells, which are broadly termed glial cells. The 3 principal types of glial cells are astrocytes, oligodendrocytes, and microglia. These glial cells can give rise to glial tumors, such as astrocytomas, oligodendrogliomas, and glioblastomas, which are among the most common primary brain tumors.When examined histologically, the neurons of the cortical gray matter demonstrate a laminar pattern. The neocortex contains 6 distinct layers, in contrast to the evolutionarily older paleocortex and archicortex, which typically contain 3 layers. The specific cytoarchitectural patterns of the cortex are not uniform throughout the cerebral cortex, and their variation was mapped by the German physician Korbinian Brodmann and presented in 1909. The so-called Brodmann areas represent cytoarchitectural differences across different brain regions, and the numbering scheme developed by Brodmann is still used to refer to distinct areas of the cortex.[4]
Layers of neocortex
- I: The molecular layer is the outermost layer of the cortex, which lies adjacent to the pial surface
- II: The external granular layer is a dense layer of primarily inhibitory granule cells; this layer serves mainly to establish intracortical connections
- III: The external pyramidal layer contains smaller neurons than its deeper counterpart; this layer provides projections to association fibers and commissural fibers.
- IV: The internal granular layer is the principal input layer of the cortex, with input derived largely from the thalamus
- V: The internal pyramidal layer is typically the largest layer within the cortex, containing large pyramidal cells; it is one of the principal output layers of the cortex, projecting to subcortical and spinal pathways; in the motor cortex, cells of this layer are termed Betz cells
- VI: The fusiform layer contains cells that form association and projection fibers
White matter
White matter tracts connect both nearby and distal brain structures and can be distinguished according to the types of connections they mediate.Projection fibers connect structures over the longest distances, such as the corticospinal projections from the motor cortex to the anterior horn cells of the spinal cord. Association fibers connect structures within the same hemisphere, such as the arcuate fasciculus, which connects the temporoparietal receptive speech areas with the frontal speech areas. Commissural fibers connect homologous structures in the left and right hemispheres, the most notable example being the corpus callosum.
Diffusion tensor imaging has emerged recently as a magnetic resonance imaging tool that provides exceptionally detailed white matter tractography in both normal and pathologic anatomy.
Glial cells
The glial cells provide supportive and regulatory functions for neurons, and in fact glial cells outnumber neurons. Three principal types of glial cells exist: microglia, astrocytes, and oligodendrocytes.Microglia have a function in the brain similar to that of the immune system. Astrocytes play a role in creating the blood-brain barrier, which allows certain substances to selectively pass from the capillary system. They are also responsible for reactive scar formation in the brain. Oligodendrocytes form myelin, which serves to electrically insulate the axons of nerve cells, allowing increased rates of conduction.
Abnormal proliferation of oligodendrocytes and astrocytes can lead to primary brain tumors called oligodendrogliomas and astrocytomas. Collectively, these belong to a family of tumors called gliomas, and the most aggressive type is termed a glioblastoma multiforme.
Functional Neuroanatomy
Our current understanding of functional localization in the cortex (see the image below) is derived from several sources, which include insights from patients with lesions involving specific areas of the cortex, awake mapping of the cortex during brain surgery, and functional imaging studies such as functional magnetic resonance imaging (MRI) and positron emission tomography (PET) in healthy volunteers. Functional localization within cerebral cortex. Some of the earliest contributions to modern language mapping can be traced to the work of neurologist Paul Broca, who studied the language deficits in patients with stroke. Broca's area, as it is termed, is a region of the frontal operculum, which also overlaps with Brodmann area 44 and 45. Three overlapping names describe this region, which is responsible for speech production. Selective damage to this region leads to difficulty speaking but typically with preserved comprehension.
Functional localization within cerebral cortex. Some of the earliest contributions to modern language mapping can be traced to the work of neurologist Paul Broca, who studied the language deficits in patients with stroke. Broca's area, as it is termed, is a region of the frontal operculum, which also overlaps with Brodmann area 44 and 45. Three overlapping names describe this region, which is responsible for speech production. Selective damage to this region leads to difficulty speaking but typically with preserved comprehension.In contrast, Wernicke's area refers to the posterior aspect of the superior temporal gyrus, which overlaps with Brodmann area 22. This region is generally responsible for speech comprehension, and selective injury to it can lead to impaired understanding with preserved speech production.
Additionally, language function is hemispherically dominant. This means that Broca's and Wernicke's aphasia typically result from damage to the hemisphere that is dominant for language. In right-handed individuals, the left hemisphere is nearly always dominant for language. However, among left-handed individuals, the left hemisphere is dominant for speech in only 70%. Bilateral representation occurs in 15% of left-handed people, and right-hemisphere language representation occurs in 15% of left-handed people.
The primary motor and sensory cortex have been mapped extensively through intraoperative stimulation in awake patients. Early work performed by neurosurgeon Wilder Penfield in Montreal led to the conceptualization of the homunculus, which is the somatotopic representation of the body in both the primary motor and primary sensory cortex (see the image below).
 Homunculus is somatotopic representation of human body in primary motor and sensory cortex. Some body parts are disproportionately represented because there are more motor and sensory fibers devoted to regions such as face and fingers. The primary motor cortex corresponds with the precentral gyrus, or Brodmann area 4. Intraoperative stimulation of the motor cortex in awake patients leads to contralateral muscle contraction in a single muscle or discrete group of muscles. The premotor cortex, which corresponds to Brodmann area 6, is also occupied with movement, but typically more complex movements are elicited by stimulation here.
Homunculus is somatotopic representation of human body in primary motor and sensory cortex. Some body parts are disproportionately represented because there are more motor and sensory fibers devoted to regions such as face and fingers. The primary motor cortex corresponds with the precentral gyrus, or Brodmann area 4. Intraoperative stimulation of the motor cortex in awake patients leads to contralateral muscle contraction in a single muscle or discrete group of muscles. The premotor cortex, which corresponds to Brodmann area 6, is also occupied with movement, but typically more complex movements are elicited by stimulation here.The primary sensory cortex corresponds with the postcentral gyrus, or Brodmann areas 1-3. The homunculus obtained from awake mapping corresponds to that of the motor cortex. Stimulation in awake patients during surgery typically leads to the subjective sensation of tingling of the corresponding body part on the opposite side of the body. Caudally, the superior parietal lobule, Brodmann areas 5 and 7, represents the secondary sensory cortex, which is felt to subserve multimodal sensory information.
The primary visual cortex corresponds to Brodmann area 17 and occupies the occipital pole. It is also termed the striate cortex. The visual cortex is retinotopically organized. Surrounding the primary visual cortex is the visual association cortex, or Brodmann areas 18 and 19.
The primary auditory cortex lies on the superior bank of the superior temporal gyrus and corresponds to Brodmann area 41. Like the primary motor, primary sensory, and visual cortices, the primary auditory cortex is tonotopically organized. The auditory association cortex, or Brodmann area 42, surrounds the primary auditory cortex.
Kaydol:
Yorumlar (Atom)